Anat Cell Biol.
2010 Sep;43(3):194-200. 10.5115/acb.2010.43.3.194.
Ethanol down regulates the expression of myelin proteolipid protein in the rat hippocampus
- Affiliations
-
- 1Department of Anatomy & Neurobiology, Institute of Health Sciences, Medical Research Center for Neural Dysfunction, Gyeongsang National University School of Medicine, Jinju, Korea. choiws@gnu.ac.kr
- 2Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- KMID: 2168874
- DOI: http://doi.org/10.5115/acb.2010.43.3.194
Abstract
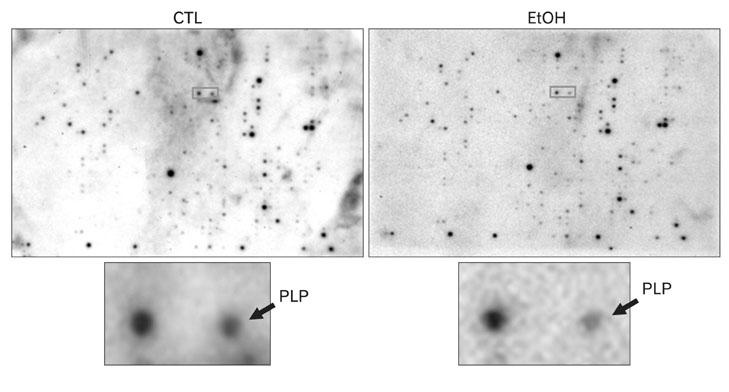
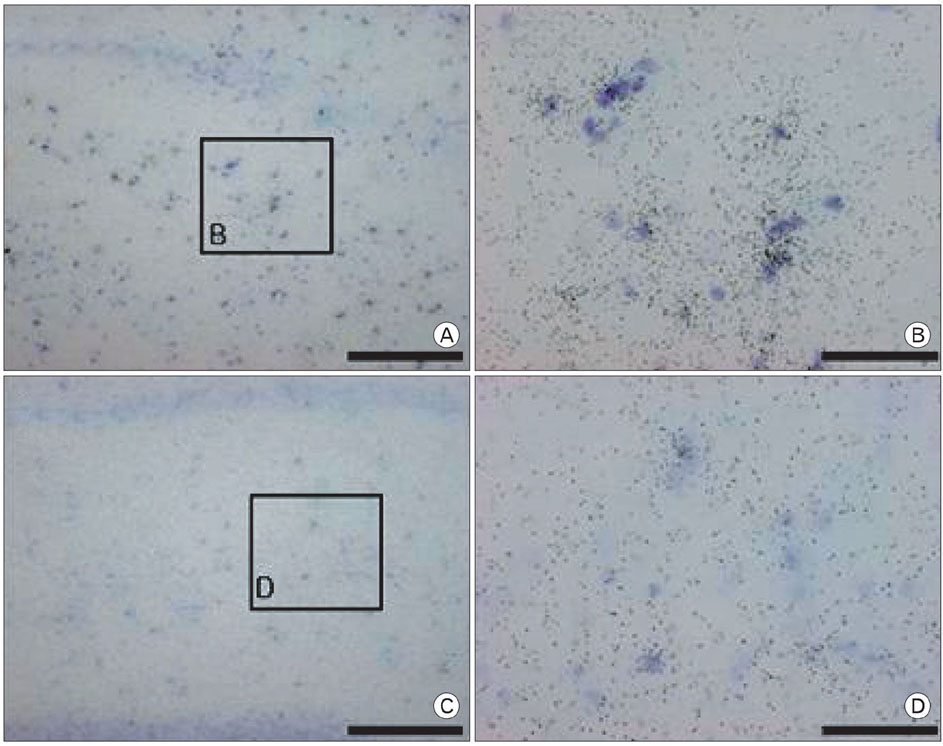
- It is well known that chronic ethanol treatment affects the synthesis of RNA and protein in the brain and the maintenance and function of nervous system. The changes in myelination-related genes are most prominent in human alcoholics. Previously, our cDNA microarray study showed altered Proteolipid protein (PLP), a major protein of central myelin. The present study aimed to gain more understanding of the expression of PLP after chronic ethanol treatment. Male Sprague-Dawley rats were daily treated with ethanol (15% in saline, 3 g/kg, i.p.) or saline for 14 days. Messenger RNAs from hippocampus of each group were subjected to cDNA expression array hybridization to determine the differential gene expressions. Among many ethanol responsive genes, PLP was negatively regulated by ethanol treatment, which is one of the most abundant proteins in the CNS and has an important role in the stabilization of myelin sheath. Using northern blot and immunohistochemical analysis, we showed the change in expression level of PLP mRNA and protein after ethanol treatment. PLP mRNA and protein were decreased in hippocampus of rat with chronic ethanol exposure, suggesting that ethanol may affect the stabilization of myelin sheath through the modulation of PLP expression and induce the pathophysiology of alcoholic brain.
Keyword
MeSH Terms
-
Alcoholics
Animals
Blotting, Northern
Brain
Chimera
DNA, Complementary
Ethanol
Gene Expression
Hippocampus
Humans
Male
Myelin Proteolipid Protein
Myelin Sheath
Nervous System
Oligonucleotide Array Sequence Analysis
Proteins
Rats
Rats, Sprague-Dawley
RNA
RNA, Messenger
DNA, Complementary
Ethanol
Myelin Proteolipid Protein
Proteins
RNA
RNA, Messenger
Figure
Reference
-
1. Boison D, Büssow H, D'Urso D, Müller HW, Stoffel W. Adhesive properties of proteolipid protein are responsible for the compaction of CNS myelin sheaths. J Neurosci. 1995. 15:5502–5513.2. Boison D, Stoffel W. Disruption of the compacted myelin sheath of axons of the central nervous system in proteolipid protein-deficient mice. Proc Natl Acad Sci U S A. 1994. 91:11709–11713.3. Bowers BJ, Radcliffe RA, Smith AM, Miyamoto-Ditmon J, Wehner JM. Microarray analysis identifies cerebellar genes sensitive to chronic ethanol treatment in PKCgamma mice. Alcohol. 2006. 40:19–33.4. Daigo M, Arai Y, Oshida K, et al. Effect of hypoxic-ischemic injury on serine palmitoyltransferase activity in the developing rat brain. Pathobiology. 2008. 75:330–334.5. Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989. 41:489–537.6. Duggan DJ, Bittner M, Chen Y, Meltzer P, Trent JM. Expression profiling using cDNA microarrays. Nat Genet. 1999. 21:1 Suppl. 10–14.7. Duncan ID, Hammang JP, Trapp BD. Abnormal compact myelin in the myelin-deficient rat: absence of proteolipid protein correlates with a defect in the intraperiod line. Proc Natl Acad Sci U S A. 1987. 84:6287–6291.8. Griffiths I, Klugmann M, Anderson T, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998. 280:1610–1613.9. Inoue Y, Sugihara Y, Nakagawa S, Shimai K. The morphological changes of oligodendroglia during the formation of myelin sheaths--Golgi study and electron microscopy. Okajimas Folia Anat Jpn. 1973. 50:327–343.10. Klugmann M, Schwab MH, Pühlhofer A, et al. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997. 18:59–70.11. Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned. Prog Neurobiol. 1999. 58:381–387.12. Lewohl JM, Dodd PR, Mayfield RD, Harris RA. Application of DNA microarrays to study human alcoholism. J Biomed Sci. 2001. 8:28–36.13. Liu J, Lewohl JM, Dodd PR, Randall PK, Harris RA, Mayfield RD. Gene expression profiling of individual cases reveals consistent transcriptional changes in alcoholic human brain. J Neurochem. 2004. 90:1050–1058.14. Möbius W, Patzig J, Nave KA, Werner HB. Phylogeny of proteolipid proteins: divergence, constraints, and the evolution of novel functions in myelination and neuroprotection. Neuron Glia Biol. 2008. 4:111–127.15. Ollat H, Sebban C. Histological lesions and changes in neurotransmitter systems in the aged brain. Discussion of their pathogenic role. Presse Med. 1983. 12:809–814.16. Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec. 1991. 229:384–398.17. Rosenbluth J, Nave KA, Mierzwa A, Schiff R. Subtle myelin defects in PLP-null mice. Glia. 2006. 54:172–182.18. Rosenbluth J, Stoffel W, Schiff R. Myelin structure in proteolipid protein (PLP)-null mouse spinal cord. J Comp Neurol. 1996. 371:336–344.19. Ryabinin AE. Role of hippocampus in alcohol-induced memory impairment: implications from behavioral and immediate early gene studies. Psychopharmacology (Berl). 1998. 139:34–43.20. Saito M, Smiley J, Toth R, Vadasz C. Microarray analysis of gene expression in rat hippocampus after chronic ethanol treatment. Neurochem Res. 2002. 27:1221–1229.21. Samson HH, Harris RA. Neurobiology of alcohol abuse. Trends Pharmacol Sci. 1992. 13:206–211.22. Sarret C, Combes P, Micheau P, Gelot A, Boespflug-Tanguy O, Vaurs-Barriere C. Novel neuronal proteolipid protein isoforms encoded by the human myelin proteolipid protein 1 gene. Neuroscience. 2010. 166:522–538.23. Tanaka H, Ma J, Tanaka KF, et al. Mice with altered myelin proteolipid protein gene expression display cognitive deficits accompanied by abnormal neuron-glia interactions and decreased conduction velocities. J Neurosci. 2009. 29:8363–8371.24. Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980. 209:711–713.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunocytochemical localization of myelin basic protein, proteolipid protein and myelin-associated glycoprotein in human oligodendrocyte in culture
- Effect of Ethanol on the Neuronal Viability and Neurite Outgrowth in Primary Cultures of Rat Hippocampus
- Cathepsin D deficiency delays central nervous system myelination by inhibiting proteolipid protein trafficking from late endosome/lysosome to plasma membrane
- Regulation of MAPK Activity by Seizure-induced MKP-1 in Rat Hippocampus
- Differential changes in the expression of cyclic nucleotide phosphodiesterase isoforms in rat brains by chronic treatment with electroconvulsive shock