Cancer Res Treat.
2007 Jun;39(2):82-87.
The Chemopreventive Effect of Retinoids on Cellular NF-kappa B Activity Induced by NMU and NEU in Human Malignant Keratinocytes
- Affiliations
-
- 1Department of Clinical Pathology, Bioindustry and Technology Research Institute, Gwangju Health College, Gwangju, Korea. kmoon@ghc.ac.kr
Abstract
-
PURPOSE: Retinoids have been shown to be effective in suppressing tumor development when chemical carcinogens such as N-nitroso-N-methylurea (NMU) and N- nitroso-N-ethylurea (NEU) were used to induce mammary tumors in a variety of animal models. However, the molecular mechanisms associated with the retinoid- mediated chemopreventive process, as linked to transcription factor NF-kappa B activation, for chemoprevention have not been elucidated. The purpose of this study was to determine the implications of NF-kappa B activation on the chemopreventive role of retinoids and their effect on cellular NF-kappa B activity that's induced by known alkylating chemical carcinogens such as NMU and NEU in human transfectant squamous cell carcinoma (SCC-13) cells.
MATERIALS AND METHODS
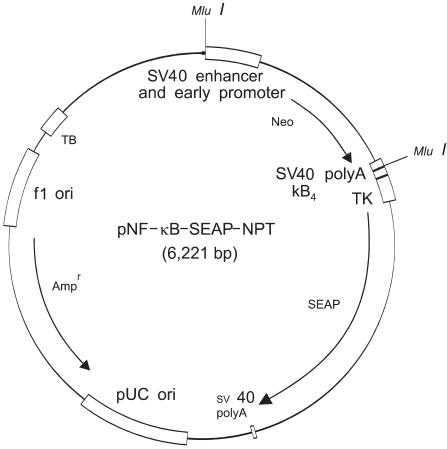
The activity of NF-kappa B, as regulated by chemical carcinogens and retinoids, was determined in cultured human SCC-13 keratinocytes that were transfected with the pNF-kappa B-SEAP-NPT plasmid; this permitted the expression of the secretory alkaline phosphatase (SEAP) reporter gene in response to the NF-kappa B activity, and the plasmid contained the neomycin phosphotransferase (NPT) gene, which confers resistance to geneticin. The reporter enzyme activity was measured using a fluorescence detection assay method.
RESULTS
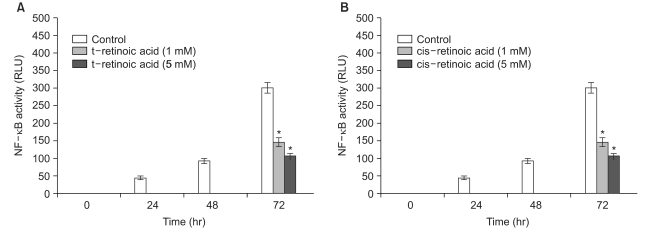
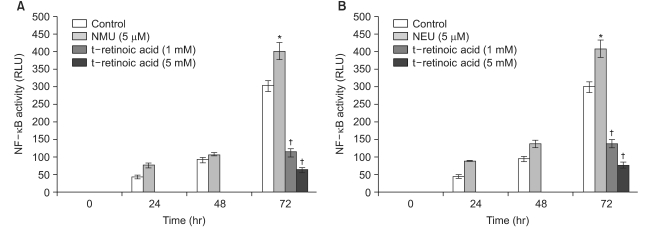
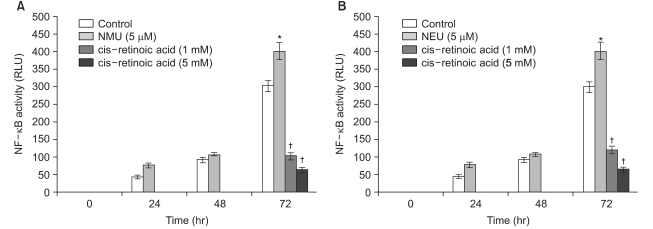
All-trans retinoic acid and 13-cis retinoic acid induced a reduction of NF-kappa B activity up to 64% and 65%, respectively, compared to the control. For the treatment of the human transfectant cells with chemical carcinogens, all-trans retinoic acid (5 mM) and 13-cis retinoic acid (5 mM) downregulated the cellular NF-kappa B activation up to 83% and 85% compared to the NF-kappa B activity that was upregulated by NMU (5 micro M) and NEU (5 micro M), respectively.
CONCLUSION
These results suggest that the chemopreventive effect of retinoids may be mediated by the down- regulated activation of NF-kappa B and that retinoids are implicated in the activation of NF-kappa B in human skin cells.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Carcinogens
Carcinoma, Squamous Cell
Chemoprevention
Fluorescence
Genes, Reporter
Humans*
Kanamycin Kinase
Keratinocytes*
Models, Animal
NF-kappa B*
Plasmids
Retinoids*
Skin
Transcription Factors
Tretinoin
Alkaline Phosphatase
Carcinogens
Kanamycin Kinase
NF-kappa B
Retinoids
Transcription Factors
Tretinoin
Figure
Reference
-
1. Baeuerle PA, Baichwal VR. NF-κB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997; 65:111–137. PMID: 9238509.2. Wulczyn FG, Krappmann D, Scheidereit C. The NF-κB/Rel and I kappa B gene families: mediators of immune response and inflammation. J Mol Med. 1996; 74:749–769. PMID: 8974017.3. Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronovo V. The NF-kappa B transcription factor and cancer: high expression of NF-kappa B-and I kappa B-related proteins in tumor cell lines. Biochem Pharmacol. 1994; 47:145–149. PMID: 8311838.4. Bours V, Bentires-Alj M, Hellin AC, Viatour P, Robe P, Delhalle S, et al. Nuclear factor-κB, cancer, and apoptosis. Biochem Pharmacol. 2000; 60:1085–1089. PMID: 11007945.
Article5. Rudkin GH, Carlsen BT, Chung CY, Huang W, Ishida K, Anvar B, et al. Retinoids inhibit squamous cell carcinoma growth and intercellular communication. J Surg Res. 2002; 103:183–189. PMID: 11922733.
Article6. Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996; 10:1031–1039. PMID: 8801164.
Article7. Lippman SM, Kessler JF, Meyskens FL Jr. Retinoids as preventive and therapeutic anticancer agents (Part I). Cancer Treat Rep. 1987; 71:391–405. PMID: 3548957.8. Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987; 330:624–629. PMID: 2825036.
Article9. Benbrook D, Lernhardt E, Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988; 333:669–672. PMID: 2836738.
Article10. Zarbl H, Sukumar S, Arthur AV, Martin-Zanca D, Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-Nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. Nature. 1985; 315:382–385. PMID: 3923365.11. Nakamura T, Ushijima T, Ishizaka Y, Nagao M, Nemoto T, Hara M, et al. Neu proto-oncogene mutation is specific for the neurofibromas in a N-nitroso-N-ethylurea-induced hamster neurofibromatosis model but not for hamster melanomas and human Schwann cell tumors. Cancer Res. 1994; 54:976–980. PMID: 7906199.12. Likhachev AJ, Ivanov MN, Bresil H, Planche-Martel G, Montesano R, Margison GP. Carcinogenicity of single doses of N-nitroso-N-methylurea and N-nitroso-N-ethylurea in syrian golden hamsters and the persistence of alkylated purines in the DNA of various tissues. Cancer Res. 1983; 43:829–833. PMID: 6848195.13. Rheinwald JG, Beckett MA. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980; 22:629–632. PMID: 6160916.
Article14. Moon KY, Hahn BS, Lee J, Kim YS. A cell-based assay system for monitoring NF-κB activity in human HaCaT transfectant cells. Anal Biochem. 2001; 292:17–21. PMID: 11319812.
Article15. Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988; 48:4827–4833. PMID: 3409223.16. Moon KY, Lee YJ, Kim YS. Upregulation of cellular NF-κB activity by alkylating carcinogens in human epidermal keratinocytes. Biol Pharm Bull. 2003; 26:1195–1197. PMID: 12913277.17. Paillaud E, Costa S, Fages C, Plassat JL, Rochette-Egly C, Monville C, et al. Retinoic acid increases proliferation rate of GL-15 glioma cells, involving activation of STAT-3 transcription factor. J Neurosci Res. 2002; 67:670–679. PMID: 11891779.
Article18. Darwiche N, Bazzi H, El-Touni L, Abou-Lteif G, Doueiri R, Hatoum A, et al. Regulation of ultraviolet B radiation-mediated activation of AP1 signaling by retinoids in primary keratinocytes. Radiat Res. 2005; 163:296–306. PMID: 15733037.
Article19. Dedieu S, Lefebvre P. Retinoids interfere with the AP1 signalling pathway in human breast cancer cells. Cell Signal. 2006; 18:889–898. PMID: 16176868.
Article20. Mestre JR, Subbaramaiah K, Sacks PG, Schantz SP, Tanabe T, Inoue H, et al. Retinoids suppress phorbol ester-mediated induction of cyclooxygenase-2. Cancer Res. 1997; 57:1081–1085. PMID: 9067275.21. Oh GS, Pae HO, Seo WG, Shin MK, Kim IK, Chai KY, et al. Inhibitory effect of retinoic acid on expression of inducible nitric oxide synthase gene in 1929 cells. Immunopharmacol Immunotoxicol. 2001; 23:335–342. PMID: 11694025.22. Moon RC, Mehta RG, Rao KVN. Sporn MB, Roberts AB, Goodman DS, editors. Retinoids and cancer in experimental animals. The Retinoids: biology, chemistry, and medicine. 1994. New York: Raven Press;p. 573–595.23. Lippman SM, Meyskens FL Jr. Treatment of advanced squamous cell carcinoma of the skin with isotretinoin. Ann Intern Med. 1987; 107:499–502. PMID: 3477106.
Article24. Koh YM, Park JS, Namkoong SE, Lee SY, Kim SA, Hong KJ, et al. Growth regulation of ovarian cancer cells through the inactivation of AP-1 by retinoid derivatives. J Korean Cancer Assoc. 2000; 32:1043–1049.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Attenuated Nuclear Factor Kappa B Activity by E7 Protein of Human Papillomavirus Type 2 in Human Epidermal Keratinocytes
- The Effect of UVB on the NF-kB and AP-1 Activity in Cultured Human Keratinocytes and Fibroblasts
- Anti-inflammatory Activity of Fucoidan with Blocking NF-kappaB and STAT1 in Human Keratinocytes Cells
- Nuclear Factor-kappaB (NF-kappaB) Activity and Levels of IL-6, IL-8 and TNF-alpha in Induced Sputum in the Exacerbation and Recovery of COPD Patients
- Effects of all-trans retinoic acid on expression of Toll-like receptor 5 on immune cells