Immune Netw.
2013 Aug;13(4):141-147. 10.4110/in.2013.13.4.141.
Cobalt Chloride-induced Hypoxia Ameliorates NLRP3-Mediated Caspase-1 Activation in Mixed Glial Cultures
- Affiliations
-
- 1Department of Microbiology, BK 21 project for Medical Science, Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul 120-752, Korea. jewookyu@yuhs.ac
- KMID: 2150779
- DOI: http://doi.org/10.4110/in.2013.13.4.141
Abstract
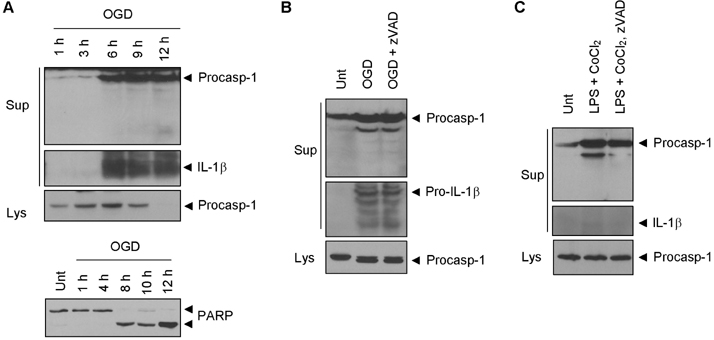
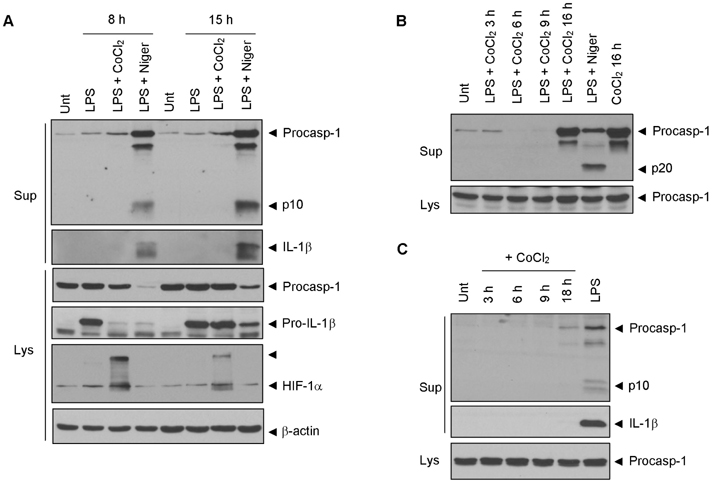
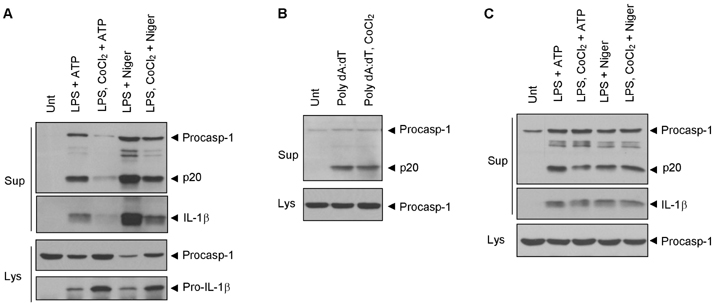
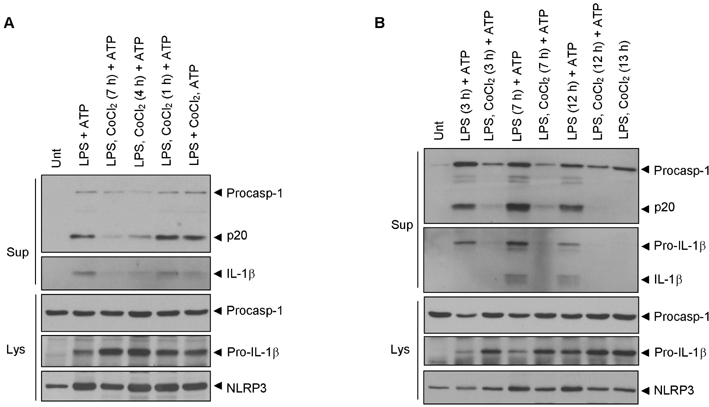
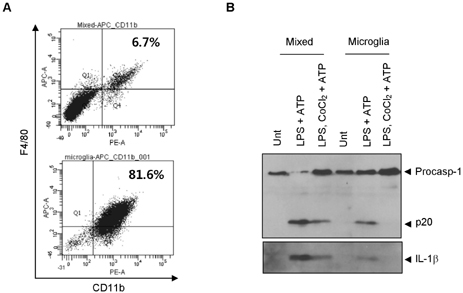
- Hypoxia has been shown to promote inflammation, including the release of proinflammatory cytokines, but it is poorly investigated how hypoxia directly affects inflammasome signaling pathways. To explore whether hypoxic stress modulates inflammasome activity, we examined the effect of cobalt chloride (CoCl2)-induced hypoxia on caspase-1 activation in primary mixed glial cultures of the neonatal mouse brain. Unexpectedly, hypoxia induced by oxygen-glucose deprivation or CoCl2 treatment failed to activate caspase-1 in microglial BV-2 cells and primary mixed glial cultures. Of particular interest, CoCl2-induced hypoxic condition considerably inhibited NLRP3-dependent caspase-1 activation in mixed glial cells, but not in bone marrow-derived macrophages. CoCl2-mediated inhibition of NLRP3 inflammasome activity was also observed in the isolated brain microglial cells, but CoCl2 did not affect poly dA:dT-triggered AIM2 inflammasome activity in mixed glial cells. Our results collectively demonstrate that CoCl2-induced hypoxia may negatively regulate NLRP3 inflammasome signaling in brain glial cells, but its physiological significance remains to be determined.
Keyword
Figure
Cited by 1 articles
-
Zika Virus Impairs Host NLRP3-mediated Inflammasome Activation in an NS3-dependent Manner
Eunji Gim, Do-Wan Shim, Inhwa Hwang, Ok Sarah Shin, Je-Wook Yu
Immune Netw. 2019;19(6):. doi: 10.4110/in.2019.19.e40.
Reference
-
1. Schroder K, Tschopp J. The inflammasomes. Cell. 2010; 140:821–832.
Article2. Hong S, Park S, Yu JW. Pyrin domain (PYD)-containing inflammasome in innate immunity. J Bacteriol Virol. 2011; 41:133–146.
Article3. Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012; 481:278–286.
Article4. Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012; 13:352–357.
Article5. Shaftel SS, Griffin WS, O'Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008; 5:7.
Article6. Meissner F, Molawi K, Zychlinsky A. Mutant superoxide dismutase 1-induced IL-1beta accelerates ALS pathogenesis. Proc Natl Acad Sci U S A. 2010; 107:13046–13050.7. Brough D, Tyrrell PJ, Allan SM. Regulation of interleukin-1 in acute brain injury. Trends Pharmacol Sci. 2011; 32:617–622.
Article8. Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008; 9:857–865.
Article9. Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013; 493:674–678.
Article10. Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011; 364:656–665.
Article11. Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009; 10:195–202.
Article12. Savage CD, Lopez-Castejon G, Denes A, Brough D. NLRP3-Inflammasome Activating DAMPs Stimulate an Inflammatory Response in Glia in the Absence of Priming Which Contributes to Brain Inflammation after Injury. Front Immunol. 2012; 3:288.
Article13. Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol. 2002; 35:67–86.
Article14. Johnson DR, O'Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system, which diabetes exacerbates. J Neurosci. 2007; 27:1161–1166.
Article15. Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, Friedlander RM. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci U S A. 2003; 100:16012–16017.
Article16. Bossenmeyer-Pourié C, Koziel V, Daval JL. Involvement of caspase-1 proteases in hypoxic brain injury. effects of their inhibitors in developing neurons. Neuroscience. 2000; 95:1157–1165.
Article17. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009; 458:509–513.
Article18. Eyo UB, Miner SA, Ahlers KE, Wu LJ, Dailey ME. P2X7 receptor activation regulates microglial cell death during oxygen-glucose deprivation. Neuropharmacology. 2013; 73C:311–319.
Article19. Almeida A, Delgado-Esteban M, Bolaños JP, Medina JM. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J Neurochem. 2002; 81:207–217.
Article20. Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011; 11:775–787.
Article21. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011; 469:221–225.
Article22. Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, McCormick M, Zhang Z, Alnemri ES. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007; 28:214–227.
Article23. Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009; 106:20388–20393.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Zika Virus Impairs Host NLRP3-mediated Inflammasome Activation in an NS3-dependent Manner
- The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout
- Extracellular Acidification Augments NLRP3-Mediated Inflammasome Signaling in Macrophages
- Interruption of Helicobacter pylori-Induced NLRP3 Inflammasome Activation by Chalcone Derivatives
- Requirement of ERK Activation in Hypoxia Induced Caspase Activation and Apoptosis of Cultured Tubular Cells