Immune Netw.
2010 Dec;10(6):212-218. 10.4110/in.2010.10.6.212.
The Stem Bark of Kalopanax pictus Exhibits Anti-inflammatory Effect through Heme Oxygenase-1 Induction and NF-kappaB Suppression
- Affiliations
-
- 1Department of Molecular Biology, College of Natural Sciences, Pusan National University, Busan 609-735, Korea. yheekim@pusan.ac.kr
- 2Department of Microbiology, College of Natural Sciences, Pusan National University, Busan 609-735, Korea.
- KMID: 2150677
- DOI: http://doi.org/10.4110/in.2010.10.6.212
Abstract
- BACKGROUND
The stem bark of Kalopanax pictus (KP) has been used in traditional medicine to treat rheumatoidal arthritis, neurotic pain and diabetes mellitus in China and Korea. In this study, the mechanism responsible for anti-inflammatory effects of KP was investigated.
METHODS
We examined the effects of KP on NO production, nitric oxide synthase (iNOS) and HO-1 expression, NF-kappaB, Nrf2 and MAPK activation in mouse peritoneal macrophages.
RESULTS
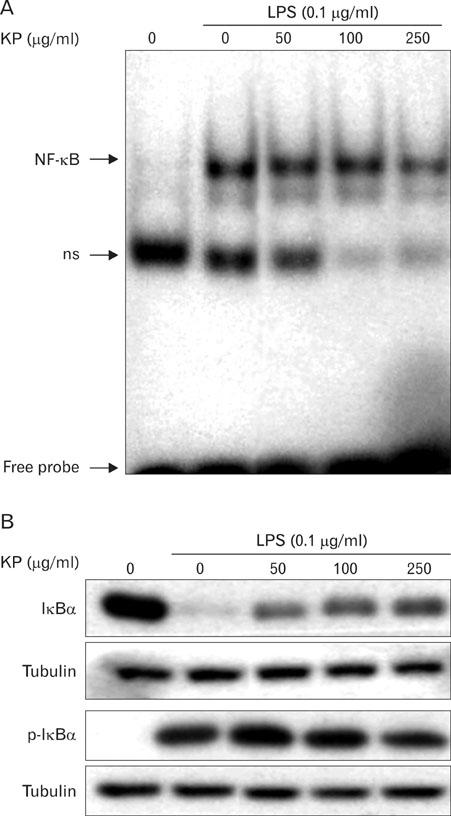
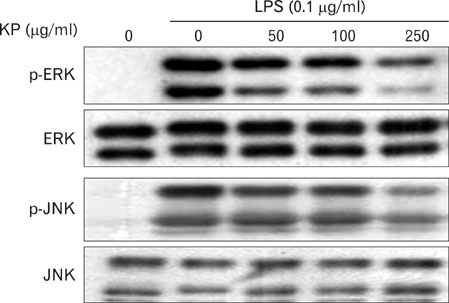
The aqueous extract of KP inhibited LPS-induced NO secretion as well as inducible iNOS expression, without affecting cell viability. KP suppressed LPS-induced NF-kappaB activation, phosphorylation and degradation of IkappaB-alpha, phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK). Furthermore, KP induced HO-1 expression and Nrf2 nuclear translocation.
CONCLUSION
These results suggest that KP has the inhibitory effects on LPS-induced NO production in macrophages through NF-kappaB suppression and HO-1 induction.
MeSH Terms
-
Animals
Arthritis
Cell Survival
China
Diabetes Mellitus
Heme
Heme Oxygenase-1
I-kappa B Proteins
JNK Mitogen-Activated Protein Kinases
Kalopanax
Korea
Macrophages
Medicine, Traditional
Mice
NF-kappa B
Nitric Oxide Synthase
Phosphorylation
Phosphotransferases
Heme
Heme Oxygenase-1
I-kappa B Proteins
JNK Mitogen-Activated Protein Kinases
NF-kappa B
Nitric Oxide Synthase
Phosphotransferases
Figure
Reference
-
1. Kim YK, Kim RG, Park SJ, Ha JH, Choi JW, Park HJ, Lee KT. In vitro antiinflammatory activity of kalopanaxsaponin A isolated from Kalopanax pictus in murine macrophage RAW 264.7 cells. Biol Pharm Bull. 2002. 25:472–476.
Article2. Li DW, Lee EB, Kang SS, Hyun JE, Whang WK. Activity-guided isolation of saponins from Kalopanax pictus with anti-inflammatory activity. Chem Pharm Bull (Tokyo). 2002. 50:900–903.
Article3. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43:109–142.4. Southan GJ, Szabó C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996. 51:383–394.
Article5. Pae HO, Chung HT. Heme oxygenase-1: its therapeutic roles in inflammatory diseases. Immune Netw. 2009. 9:12–19.
Article6. Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000. 6:422–428.
Article7. Suh GY, Jin Y, Yi AK, Wang XM, Choi AM. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol. 2006. 35:220–226.
Article8. Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004. 18:765–767.
Article9. Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, Otterbein LE. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L1131–L1137.
Article10. Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010. 80:1895–1903.
Article11. Kundu JK, Surh YJ. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res. 2010. 27:999–1013.
Article12. Kim Y, Moon JS, Lee KS, Park SY, Cheong J, Kang HS, Lee HY, Kim HD. Ca2+/calmodulin-dependent protein phosphatase calcineurin mediates the expression of iNOS through IKK and NF-kappaB activity in LPS-stimulated mouse peritoneal macrophages and RAW 264.7 cells. Biochem Biophys Res Commun. 2004. 314:695–703.
Article13. Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006. 25:6731–6748.
Article14. Lee KT, Sohn IC, Park HJ, Kim DW, Jung GO, Park KY. Essential moiety for antimutagenic and cytotoxic activity of hederagenin monodesmosides and bisdesmosides isolated from the stem bark of Kalopanax pictus. Planta Med. 2000. 66:329–332.
Article15. Li DW, Hyun JE, Jeong CS, Kim YS, Lee EB. Antiinflammatory activity of alpha-hederin methyl ester from the alkaline hydrolysate of the butanol fraction of Kalopanax pictus bark extract. Biol Pharm Bull. 2003. 26:429–433.
Article16. Choi J, Huh K, Kim SH, Lee KT, Kwon SH, Park HJ. Toxicology of Kalopanax pictus extract and hematological effect of the isolated anti-rheumatoidal kalopanaxsaponin A on the Freunds complete adjuvant reagent-treated rat. Arch Pharm Res. 2001. 24:119–125.
Article17. Choi J, Huh K, Kim SH, Lee KT, Lee HK, Park HJ. Kalopanaxsaponin A from Kalopanax pictus, a potent antioxidant in the rheumatoidal rat treated with Freund's complete adjuvant reagent. J Ethnopharmacol. 2002. 79:113–118.
Article18. Choi J, Huh K, Kim SH, Lee KT, Park HJ, Han YN. Antinociceptive and anti-rheumatoidal effects of Kalopanax pictus extract and its saponin components in experimental animals. J Ethnopharmacol. 2002. 79:199–204.
Article19. Kim DH, Bae EA, Han MJ, Park HJ, Choi JW. Metabolism of kalopanaxsaponin K by human intestinal bacteria and antirheumatoid arthritis activity of their metabolites. Biol Pharm Bull. 2002. 25:68–71.
Article20. Park HJ, Kwon SH, Lee JH, Lee KH, Miyamoto K, Lee KT. Kalopanaxsaponin A is a basic saponin structure for the anti-tumor activity of hederagenin monodesmosides. Planta Med. 2001. 67:118–121.
Article21. Park HJ, Kim DH, Choi JW, Park JH, Han YN. A potent anti-diabetic agent from Kalopanax pictus. Arch Pharm Res. 1998. 21:24–29.22. Kim DW, Bang KH, Rhee YH, Lee KT, Park HJ. Growth inhibitory activities of kalopanaxsaponins A and I against human pathogenic fungi. Arch Pharm Res. 1998. 21:688–691.
Article23. Kim IT, Park YM, Shin KM, Ha J, Choi J, Jung HJ, Park HJ, Lee KT. Anti-inflammatory and anti-nociceptive effects of the extract from Kalopanax pictus, Pueraria thunbergiana and Rhus verniciflua. J Ethnopharmacol. 2004. 94:165–173.
Article24. Vareille M, Rannou F, Thélier N, Glasser AL, de Sablet T, Martin C, Gobert AP. Heme oxygenase-1 is a critical regulator of nitric oxide production in enterohemorrhagic Escherichia coli-infected human enterocytes. J Immunol. 2008. 180:5720–5726.
Article25. Takagi T, Naito Y, Uchiyama K, Yoshikawa T. The role of heme oxygenase and carbon monoxide in inflammatory bowel disease. Redox Rep. 2010. 15:193–201.
Article26. Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, Sodhi K, Canestraro M, Martasek P, Peterson SJ, Kappas A, Abraham NG. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension. 2010. 56:1124–1130.
Article27. Yin H, Li X, Gong Q, Jin X, Gu H, Yuan B, Zhang B, Zheng F, Gong F, Zhu J. Heme oxygenase-1 upregulation improves lipopolysaccharide-induced acute lung injury involving suppression of macrophage migration inhibitory factor. Mol Immunol. 2010. 47:2443–2449.
Article28. Wu ML, Ho YC, Yet SF. A central role of heme oxygenase-1 in cardiovascular protection. Antioxid Redox Signal. 2010. [Epub ahead of print].
Article29. Mandal P, Pritchard MT, Nagy LE. Anti-inflammatory pathways and alcoholic liver disease: role of an adiponectin/interleukin-10/heme oxygenase-1 pathway. World J Gastroenterol. 2010. 16:1330–1336.
Article30. Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet SF, Lee ME, Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1-deficient mice. Circulation. 2000. 102:3015–3022.
Article31. Radhakrishnan N, Yadav SP, Sachdeva A, Pruthi PK, Sawhney S, Piplani T, Wada T, Yachie A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J Pediatr Hematol Oncol. 2011. 33:74–78.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rebamipide Protects TNBS Induced Colonic Damage Through Down-regulation of NF-kappaB Activation and Induction of Heme Oxygenase -1 Expression
- HO-1 Reduces the Severity of Trinitrobenzene Sulfonic Acid-induced Colitis through Suppression of NF-kappaB Activation
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- The Anti-Inflammatory Activity of Eucommia ulmoides Oliv. Bark. Involves NF-κB Suppression and Nrf2-Dependent HO-1 Induction in BV-2 Microglial Cells
- Suppression of Heme Oxygenase-1 by Prostaglandin E2-Protein Kinase A-A-Kinase Anchoring Protein Signaling Is Central for Augmented Cyclooxygenase-2 Expression in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages