Immune Netw.
2015 Dec;15(6):291-303. 10.4110/in.2015.15.6.291.
The Anti-inflammatory Effect of GV1001 Mediated by the Downregulation of ENO1-induced Pro-inflammatory Cytokine Production
- Affiliations
-
- 1Laboratory of Vitamin C and Antioxidant Immunology, Department of Anatomy, Seoul National University College of Medicine, Seoul 03080, Korea. genius29@snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul 03080, Korea.
- 3Department of Endodontics, Seoul National University School of Dentistry, Seoul 03080, Korea.
- 4Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology and College of Medicine, Medical Research Center, Seoul National University, Seoul 03080, Korea.
- 5Division of Rheumatology, Department of Internal Medicine, College of Medicine, Seoul National University, Seoul 03080, Korea.
- KMID: 2132703
- DOI: http://doi.org/10.4110/in.2015.15.6.291
Abstract
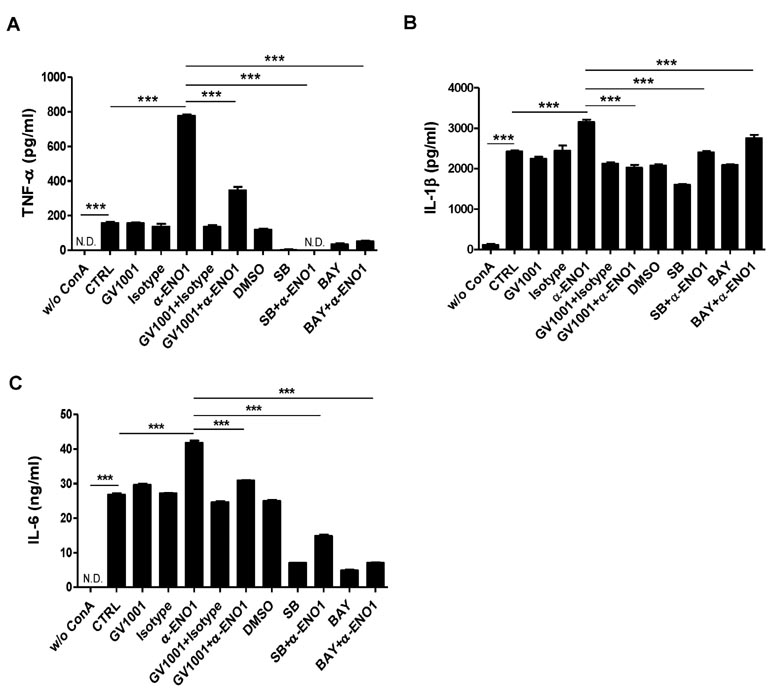
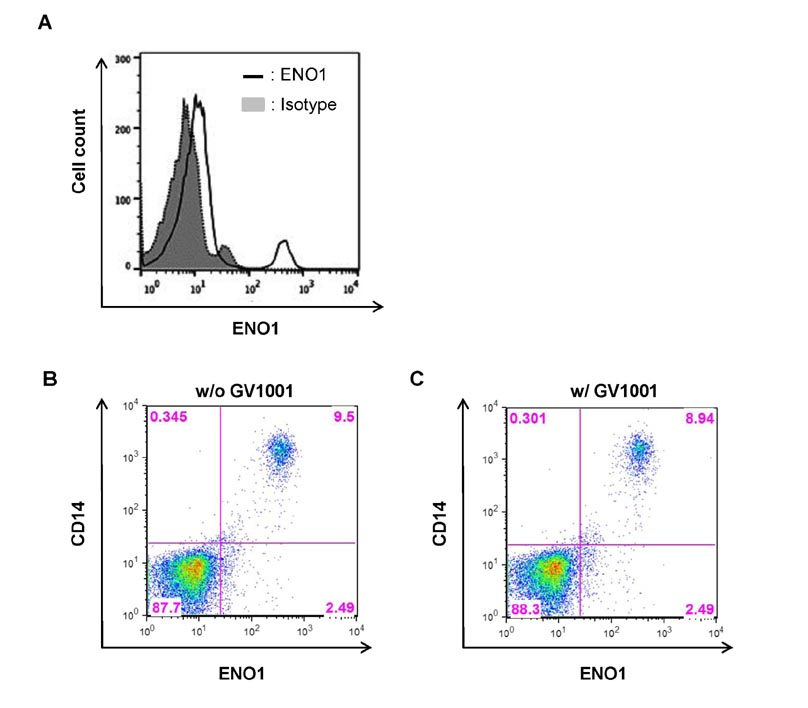
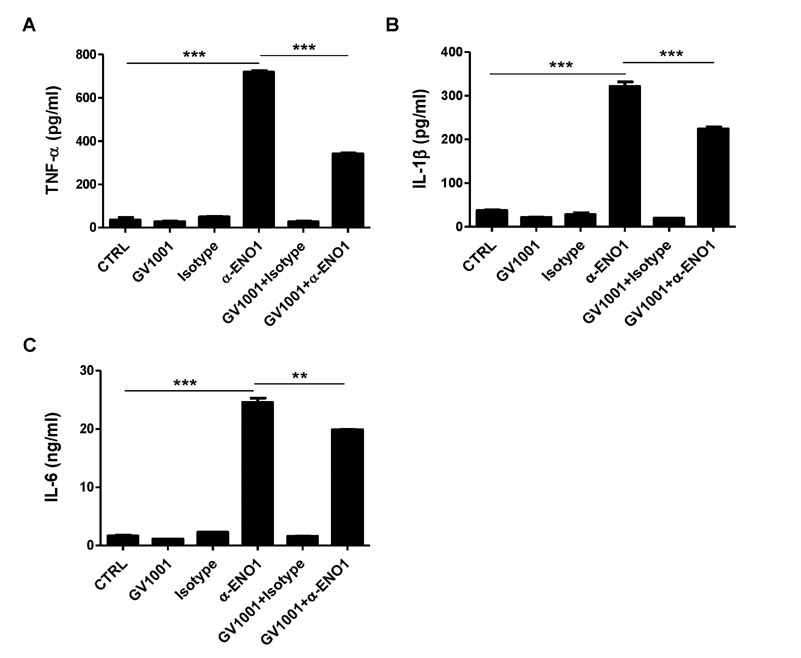
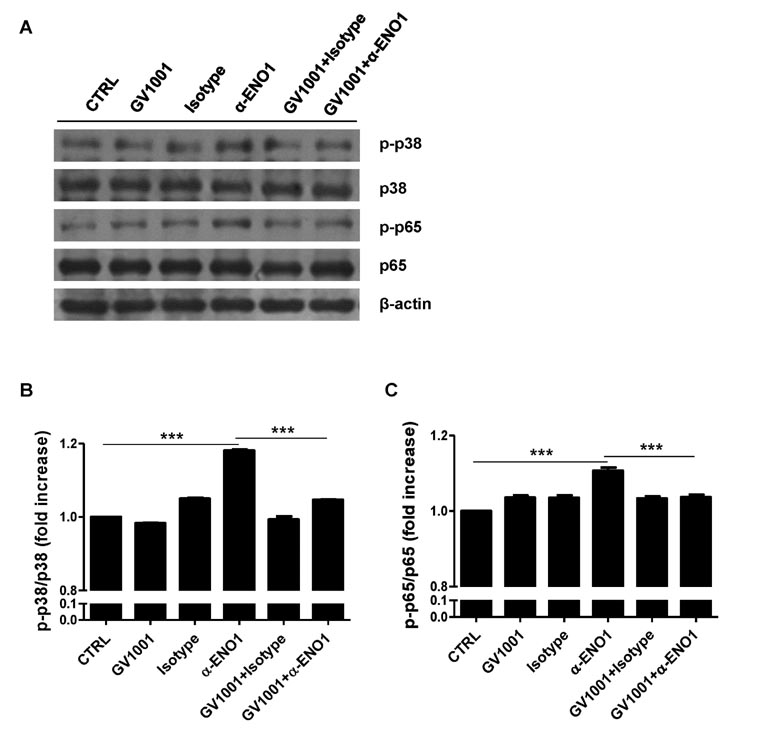
- GV1001 is a peptide derived from the human telomerase reverse transcriptase (hTERT) sequence that is reported to have anti-cancer and anti-inflammatory effects. Enolase1 (ENO1) is a glycolytic enzyme, and stimulation of this enzyme induces high levels of pro-inflammatory cytokines from concanavalin A (Con A)-activated peripheral blood mononuclear cells (PBMCs) and ENO1-expressing monocytes in healthy subjects, as well as from macrophages in rheumatoid arthritis (RA) patients. Therefore, this study investigated whether GV1001 downregulates ENO1-induced pro-inflammatory cytokines as an anti-inflammatory peptide. The results showed that GV1001 does not affect the expression of ENO1 in either Con A-activated PBMCs or RA PBMCs. However, ENO1 stimulation increased the production of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, and IL-6, and these cytokines were downregulated by pretreatment with GV1001. Moreover, p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-kappaB were activated when ENO1, on the surface of Con A-activated PBMCs and RA PBMCs, was stimulated, and they were successfully suppressed by pre-treatment with GV1001. These results suggest that GV1001 may be an effective anti-inflammatory peptide that downregulates the production of pro-inflammatory cytokines through the suppression of p38 MAPK and NF-kappaB activation following ENO1 stimulation.
Keyword
MeSH Terms
-
Arthritis, Rheumatoid
Concanavalin A
Cytokines
Down-Regulation*
Humans
Inflammation
Interleukin-6
Interleukins
Macrophages
Monocytes
NF-kappa B
p38 Mitogen-Activated Protein Kinases
Protein Kinases
Telomerase
Tumor Necrosis Factor-alpha
Concanavalin A
Cytokines
Interleukin-6
Interleukins
NF-kappa B
Protein Kinases
Telomerase
Tumor Necrosis Factor-alpha
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, Cunningham D, Falk S, Wadd N, Harrison M, Corrie P, Iveson T, Robinson A, McAdam K, Eatock M, Evans J, Archer C, Hickish T, Garcia-Alonso A, Nicolson M, Steward W, Anthoney A, Greenhalf W, Shaw V, Costello E, Naisbitt D, Rawcliffe C, Nanson G, Neoptolemos J. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014; 15:829–840.
Article2. Staff C, Mozaffari F, Frodin JE, Mellstedt H, Liljefors M. Telomerase (GV1001) vaccination together with gemcitabine in advanced pancreatic cancer patients. Int J Oncol. 2014; 45:1293–1303.
Article3. Annels NE, Shaw VE, Gabitass RF, Billingham L, Corrie P, Eatock M, Valle J, Smith D, Wadsley J, Cunningham D, Pandha H, Neoptolemos JP, Middleton G. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer. Cancer Immunol Immunother. 2014; 63:175–183.
Article4. Brunsvig PF, Kyte JA, Kersten C, Sundstrom S, Moller M, Nyakas M, Hansen GL, Gaudernack G, Aamdal S. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011; 17:6847–6857.
Article5. Hunger RE, Kernland Lang K, Markowski CJ, Trachsel S, Moller M, Eriksen JA, Rasmussen AM, Braathen LR, Gaudernack G. Vaccination of patients with cutaneous melanoma with telomerase-specific peptides. Cancer Immunol Immunother. 2011; 60:1553–1564.
Article6. Lee SA, Kim BR, Kim BK, Kim DW, Shon WJ, Lee NR, Inn KS, Kim BJ. Heat shock protein-mediated cell penetration and cytosolic delivery of macromolecules by a telomerase-derived peptide vaccine. Biomaterials. 2013; 34:7495–7505.
Article7. Koo TY, Yan JJ, Yang J. Protective effect of peptide GV1001 against renal ischemia-reperfusion injury in mice. Transplant Proc. 2014; 46:1117–1122.
Article8. Greten TF, Forner A, Korangy F, N'Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010; 10:209.
Article9. Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, Plow EF. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry. 1991; 30:1682–1691.
Article10. Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995; 227:407–415.
Article11. Lopez-Alemany R, Longstaff C, Hawley S, Mirshahi M, Fabregas P, Jardi M, Merton E, Miles LA, Felez J. Inhibition of cell surface mediated plasminogen activation by a monoclonal antibody against alpha-Enolase. Am J Hematol. 2003; 72:234–242.12. Wygrecka M, Marsh LM, Morty RE, Henneke I, Guenther A, Lohmeyer J, Markart P, Preissner KT. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009; 113:5588–5598.
Article13. Giallongo A, Feo S, Showe LC, Croce CM. Isolation and partial characterization of a 48-kDa protein which is induced in normal lymphocytes upon mitogenic stimulation. Biochem Biophys Res Commun. 1986; 134:1238–1244.
Article14. Felez J, Miles LA, Plescia J, Plow EF. Regulation of plasminogen receptor expression on human monocytes and monocytoid cell lines. J Cell Biol. 1990; 111:1673–1683.
Article15. Bae S, Kim H, Lee N, Won C, Kim HR, Hwang YI, Song YW, Kang JS, Lee WJ. alpha-Enolase expressed on the surfaces of monocytes and macrophages induces robust synovial inflammation in rheumatoid arthritis. J Immunol. 2012; 189:365–372.
Article16. Saulot V, Vittecoq O, Charlionet R, Fardellone P, Lange C, Marvin L, Machour N, Le Loet X, Gilbert D, Tron F. Presence of autoantibodies to the glycolytic enzyme alpha-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheum. 2002; 46:1196–1201.
Article17. Mosca M, Chimenti D, Pratesi F, Baldini C, Anzilotti C, Bombardieri S, Migliorini P. Prevalence and clinico-serological correlations of anti-alpha-enolase, anti-C1q, and antidsDNA antibodies in patients with systemic lupus erythematosus. J Rheumatol. 2006; 33:695–697.18. Wakui H, Imai H, Komatsuda A, Miura AB. Circulating antibodies against alpha-enolase in patients with primary membranous nephropathy (MN). Clin Exp Immunol. 1999; 118:445–450.
Article19. Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004; 36:372–378.
Article20. Tak PP, van der Lubbe A, Cauli A, Daha MR, Smeets TJ, Kluin PM, Meinders AE, Yanni G, Panayi GS, Breedveld FC. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum. 1995; 38:1457–1465.
Article21. Ishikawa H, Hirata S, Andoh Y, Kubo H, Nakagawa N, Nishibayashi Y, Mizuno K. An immunohistochemical and immunoelectron microscopic study of adhesion molecules in synovial pannus formation in rheumatoid arthritis. Rheumatol Int. 1996; 16:53–60.
Article22. Furuzawa-Carballeda J, Macip-Rodriguez PM, Cabral AR. Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. Clin Exp Rheumatol. 2008; 26:554–560.23. Xue C, Takahashi M, Hasunuma T, Aono H, Yamamoto K, Yoshino S, Sumida T, Nishioka K. Characterisation of fibroblast-like cells in pannus lesions of patients with rheumatoid arthritis sharing properties of fibroblasts and chondrocytes. Ann Rheum Dis. 1997; 56:262–267.
Article24. Robinson DR, Tashjian AH Jr., Levine L. Prostaglandin-stimulated bone resorption by rheumatoid synovia. A possible mechanism for bone destruction in rheumatoid arthritis. J Clin Invest. 1975; 56:1181–1188.
Article25. Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, Takahashi K, Furuya T, Ishiyama S, Kim KJ, Saito S, Nishikawa T, Takahashi N, Togari A, Tomatsu T, Suda T, Kamatani N. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001; 44:1003–1012.
Article26. Van Boxel JA, Paget SA. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975; 293:517–520.
Article27. Slauenwhite D, Gebremeskel S, Doucette CD, Hoskin DW, Johnston B. Regulation of cytokine polarization and T cell recruitment to inflamed paws in mouse collagen-induced arthritis by the chemokine receptor CXCR6. Arthritis Rheumatol. 2014; 66:3001–3012.
Article28. Brennan F, Beech J. Update on cytokines in rheumatoid arthritis. Curr Opin Rheumatol. 2007; 19:296–301.
Article29. Firestein GS. Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis. J Clin Invest. 1999; 103:3–4.
Article30. Moore TL, Gillian BE, Crespo-Pagnussat S, Feller L, Chauhan AK. Measurement and evaluation of isotypes of anti-citrullinated fibrinogen and anti-citrullinated alpha-enolase antibodies in juvenile idiopathic arthritis. Clin Exp Rheumatol. 2014; 32:740–746.31. Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010; 62:1576–1582.
Article32. Miossec P. An update on the cytokine network in rheumatoid arthritis. Curr Opin Rheumatol. 2004; 16:218–222.
Article33. Matsukawa A, Yoshimura T, Miyamoto K, Ohkawara S, Yoshinaga M. Analysis of the inflammatory cytokine network among TNF alpha, IL-1 beta, IL-1 receptor antagonist, and IL-8 in LPS-induced rabbit arthritis. Lab Invest. 1997; 76:629–638.34. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007; 7:429–442.
Article35. Jue DM, Jeon KI, Jeong JY. Nuclear factor kappaB (NF-kappaB) pathway as a therapeutic target in rheumatoid arthritis. J Korean Med Sci. 1999; 14:231–238.
Article36. Ivashkiv LB, Hu X. The JAK/STAT pathway in rheumatoid arthritis: pathogenic or protective? Arthritis Rheum. 2003; 48:2092–2096.37. Isomaki P, Junttila I, Vidqvist KL, Korpela M, Silvennoinen O. The activity of JAK-STAT pathways in rheumatoid arthritis: constitutive activation of STAT3 correlates with interleukin 6 levels. Rheumatology (Oxford). 2015; 54:1103–1113.
Article38. Campbell L, Chen C, Bhagat SS, Parker RA, Ostor AJ. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford). 2011; 50:552–562.
Article39. Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol. 2008; 151–160.
Article40. Cohen G, Courvoisier N, Cohen JD, Zaltni S, Sany J, Combe B. The efficiency of switching from infliximab to etanercept and vice-versa in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2005; 23:795–800.41. Makarov SS. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001; 3:200–206.42. Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford). 2008; 47:584–590.
Article43. Clark AR, Dean JL. The p38 MAPK Pathway in Rheumatoid Arthritis: A Sideways Look. Open Rheumatol J. 2012; 6:209–219.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acrosorium polyneurum Extract Inhibits the LPS-Induced Inflammatory Response by Impairing the MAPK and NF-κB Pathways
- Anti-inflammatory Activity of Carpinus tschonoskii Leaves Extract in R848-stimulated Bone Marrow-derived Macrophages and Dendritic Cells
- Role of Gallic Acid in Inflammatory Allergic Process
- Suppressed Production of Pro-inflammatory Cytokines by LPS-Activated Macrophages after Treatment with Toxoplasma gondii Lysate
- Differential Modulation of Lipopolysaccharide-Induced Inflammatory Cytokine Production by and Antioxidant Activity of Fomentariol in RAW264.7 Cells