Korean Circ J.
2007 Sep;37(9):408-413. 10.4070/kcj.2007.37.9.408.
The Cardioprotective Effects of Resveratrol via Anti-Apoptosis in Hypoxic Injury of Myocardial Cells
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, The Catholic University of Daegu, Daegu, Korea. grapfarm@hanmail.net
- 2Department of Pediatrics, Dongguk University College of Medicine, Gyeongju, Korea.
- 3Department of Opthalmology, Dongguk University College of Medicine, Gyeongju, Korea.
- KMID: 2093976
- DOI: http://doi.org/10.4070/kcj.2007.37.9.408
Abstract
-
BACKGROUND AND OBJECTIVES: Resveratrol (trans-3, 4', 5-trihydroxy-stilbene), a naturally occurring polyphenolic phytoalexin found abundantly in grape skins and red wines, has been reported to protect heart cells from ischemia/reperfusion (I/R) injury through its significant antioxidant properties. Apoptosis of cardiac myocytes is also involved in several cardiovascular diseases, but it remains unknown whether the protective effects of resveratrol in hypoxic myocardial cell injury are mediated via suppression of apoptosis. In this study, we investigated whether resveratrol confers cardioprotection against hypoxia via anti-apoptosis in a hypoxic model of cultured H9c2 cardiomyoblasts.
MATERIALS AND METHODS
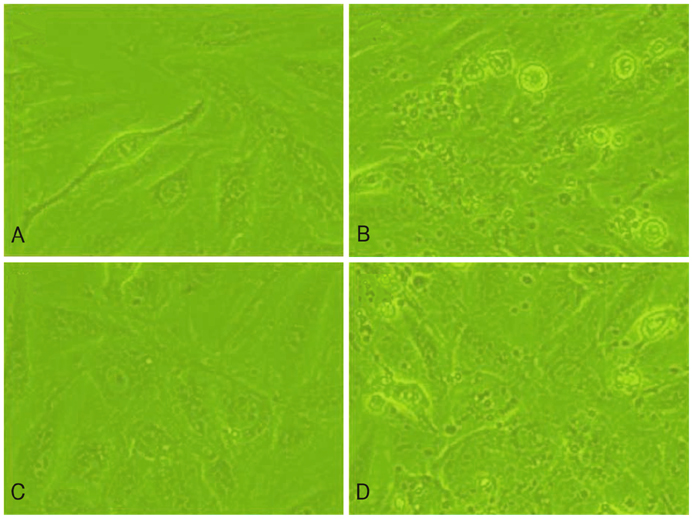
H9c2 cardiomyoblasts were obtained from the Korean Cell Line Bank. The cultured cells were divided into four groups: a normal control group, a hypoxia group, a group treated with resveratrol (10 microgram/mL) before hypoxic insult, and a group treated with resveratrol (10 microgram/mL) after hypoxic insult. The control group was placed in 5% CO2 incubators, and the hypoxia and resveratrol-treated groups were placed in 1% O2 incubators. Apoptosis was assayed by cytological analysis with Western blotting and real-time PCR for Bcl-2, Bax, and caspase-3.
RESULTS
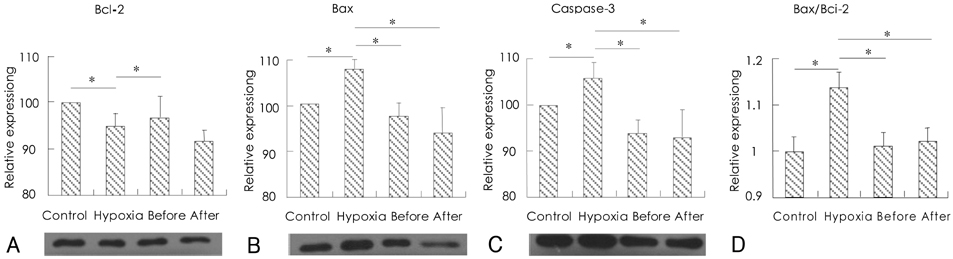
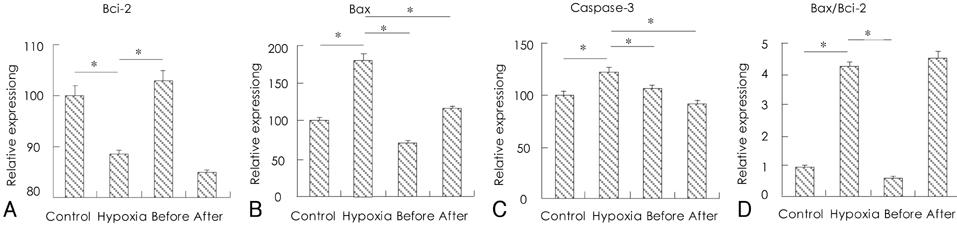
The expression of Bcl-2 was significantly decreased in the hypoxia group compared with the control group, and resveratrol treatment inhibited the hypoxia-induced decline of Bcl-2 in hypoxic myocardial cells. Conversely, the expressions of Bax and caspase-3 were significantly increased in the hypoxia group, while resveratrol inhibited the hypoxia-induced increase of Bax and caspase. In addition, hypoxia significantly increased the ratio of Bax/Bcl-2 expression, but it was significantly decreased in the resveratrol-treated group.
CONCLUSION
The present study demonstrates that the cardioprotective effects of resveratrol in hypoxic injury are mediated via the mechanisms of anti-apoptosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992. 339:1523–1526.2. Kopp P. Resveratrol, a phytoestrogen found in red wine: a possible explanation for the conundrum of the 'French paradox'? Eur J Endocrinol. 1998. 138:619–620.3. Cao Z, Li Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur J Pharmacol. 2004. 489:39–48.4. Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995. 235:207–219.5. Khan NQ, Lees DM, Douthwaite JA, Carrier MJ, Corder R. Comparison of red wine extract and polyphenol constituents on endothelin-1 synthesis by cultured endothelial cells. Clin Sci. 2002. 103:Suppl 48. 72S–75S.6. Naderali EK, Doyle PJ, Williams G. Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female guineapigs. Clin Sci. 2000. 98:537–543.7. Das S, Cordis GA, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor activation. Am J Physiol Heart Circ Physiol. 2005. 288:H328–H335.8. Giovannini L, Migliori M, Longoni BM, et al. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol. 2001. 37:262–270.9. Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK. Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci. 2001. 69:1057–1065.10. Ray PS, Maulik G, Cordis GA, Bertelli AA, Bertelli A, Das DK. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med. 1999. 27:160–169.11. Bradamante S, Barenghi L, Piccinini F, et al. Resveratrol provides late-phase cardioprotection by means of a nitric oxide-and adenosine-mediated mechanism. Eur J Pharmacol. 2003. 465:115–123.12. Lee SH, Kim YD. Death and survival of cardiomyocytes in acute ischemia. Korean Circ J. 2006. 36:165–177.13. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972. 26:239–257.14. Kim YK, Han DS, Lee MY, et al. Apoptosis in ischemia-reperfused myocardiaum of rabbit. Korean Circ J. 1997. 27:1017–1026.15. Yaoita H, Ogawa K, Maehara K, Maruyama Y. Apoptosis in relevant clinical situations: contribution of apoptosis in myocardial infarction. Cardiovasc Res. 2000. 45:630–641.16. Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003. 92:139–150.17. Morin C, Zini R, Albengres E, Bertelli AA, Bertelli A, Tillement JP. Evidence for resveratrol-induced preservation of brain mitochondria functions after hypoxia-reoxygenation. Drugs Exp Clin Res. 2003. 29:227–233.18. Dore S. Unique properties of polyphenol stilbenes in the brain: more than direct antioxidant actions; gene/protein regulatory activity. Neurosignals. 2005. 14:61–70.19. Seo HS. Apoptosis in cardiovascular system. Korean Circ J. 1997. 27:793–799.20. Joza N, Kroemer G, Penninger JM. Genetic analysis of the mammalian cell death machinery. Trends Genet. 2002. 18:142–149.21. Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998. 281:1305–1308.22. Tatsumi T, Shiraishi J, Keira N, et al. Intracellular ATP is required for mitochondrial apoptotic pathways in isolated hypoxic rat cardiac myocytes. Cardiovasc Res. 2003. 59:428–440.23. Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997. 275:1129–1132.24. Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996. 94:1506–1512.25. Alnemri ES, Livingston DJ, Nicholson DW, et al. Human ICE / CED-3 protease nomenclature. Cell. 1996. 87:171.26. Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly (ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993. 53:3976–3985.27. Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001. 2:589–598.28. de Moissac D, Gurevich RM, Zheng H, Singal PK, Kirshenbaum LA. Caspase activation and mitochondrial cytochrome C release during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol. 2000. 32:53–63.29. Jung F, Weiland U, Johns RA, Ihling C, Dimmeler S. Chronic hypoxia induces apoptosis in cardiac myocytes: a possible role for Bcl-2-like proteins. Biochem Biophys Res Commun. 2001. 286:419–425.30. Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res. 2003. 13:385–391.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Nitric Oxide Synthase (NOS) Isoforms in relation to Resveratrol Administration in Hypoxic Injury of Myocardial Cells
- Neuroprotective effects of resveratrol via anti-apoptosis on hypoxic-ischemic brain injury in neonatal rats
- Resveratrol at High Doses Acts as an Apoptotic Inducer in Endothelial Cells
- Flow Cytometric Analysis of the Effects of Resveratrol on the Survival of Human Tennon's Capsule Fibroblasts
- Resveratrol attenuates aging-induced mitochondrial dysfunction and mitochondria-mediated apoptosis in the rat heart