J Korean Ophthalmol Soc.
2015 Aug;56(8):1268-1273. 10.3341/jkos.2015.56.8.1268.
Flow Cytometric Analysis of the Effects of Resveratrol on the Survival of Human Tennon's Capsule Fibroblasts
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2215081
- DOI: http://doi.org/10.3341/jkos.2015.56.8.1268
Abstract
- PURPOSE
Resveratrol exerts cytoprotective or cytotoxic effects according to cell type. This study was performed to evaluate the effects of resveratrol on the survival of cultured human Tenon's capsule fibroblasts (HTFBs).
METHODS
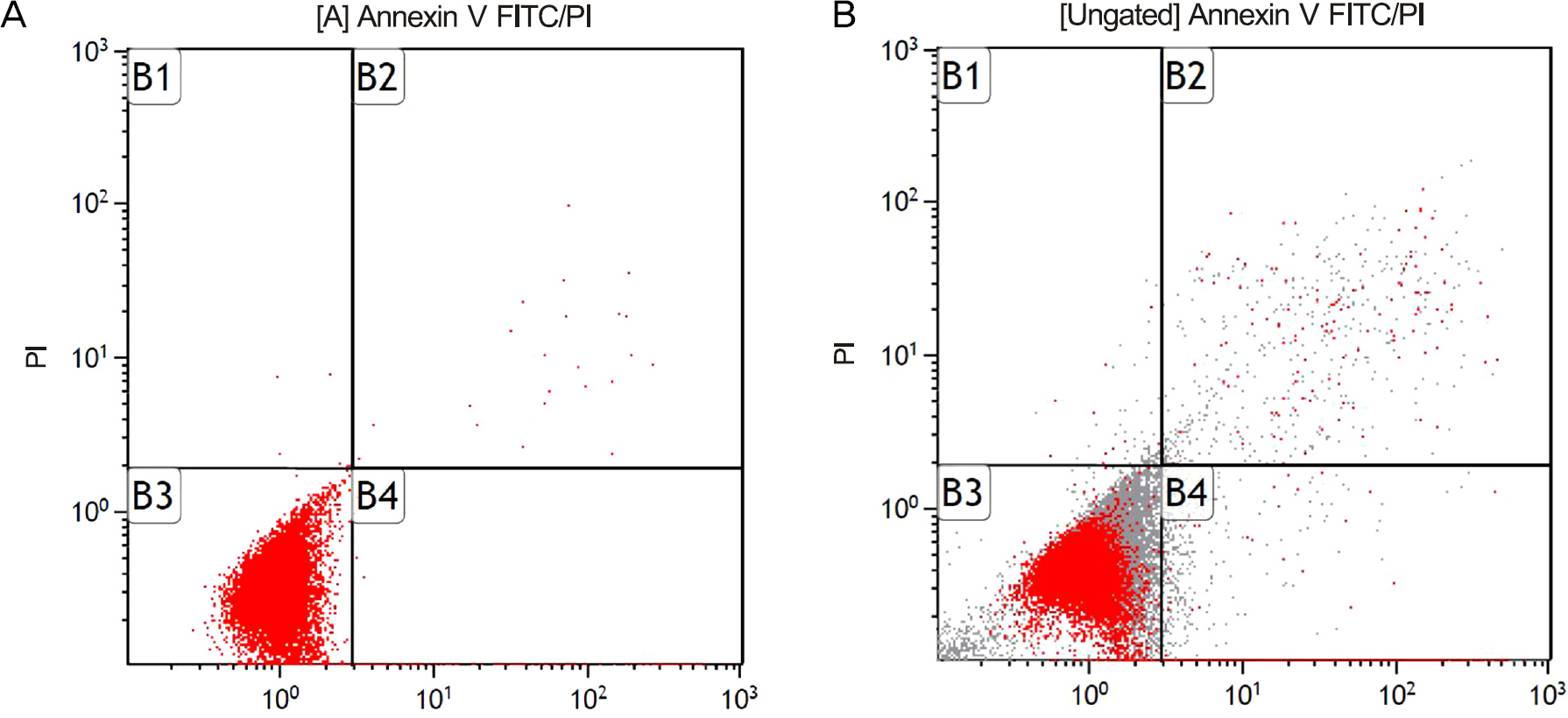
Primarily cultured HTFBs were exposed to 0, 10, or 100 microM resveratrol for 3 days. Cellular survival was assessed using the MTT assay and degree of apoptosis was analyzed with flow cytometry using annexin-V/propidium iodide double staining.
RESULTS
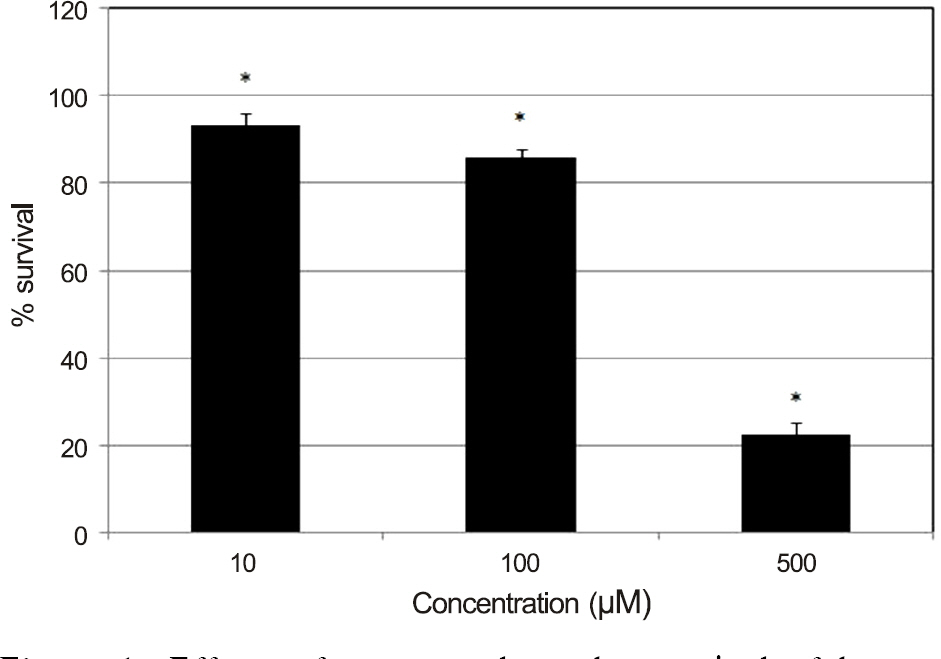
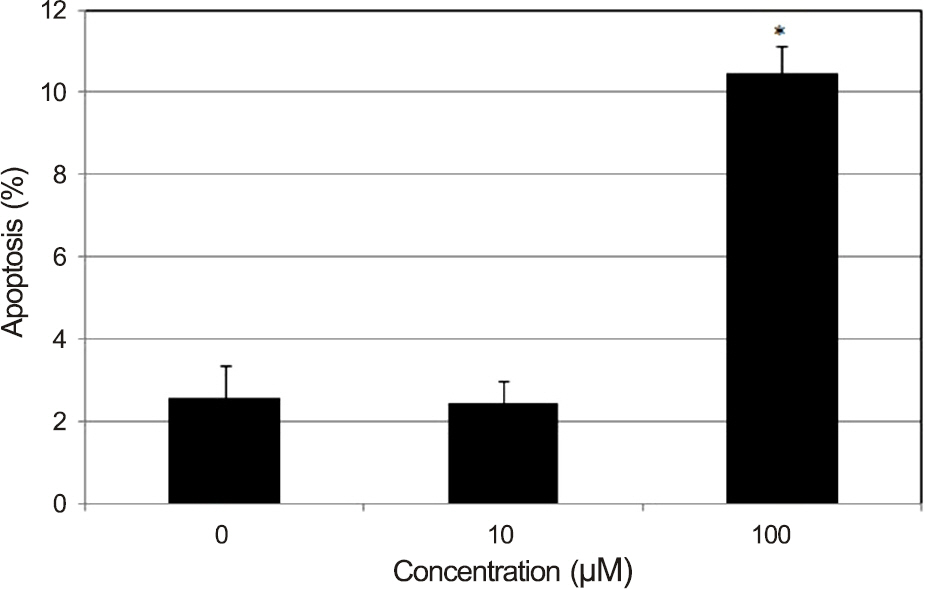
Resveratrol decreased the survival of HTFBs after exposure to 10 microM (p = 0.04). In flow cytometric analysis, 10 microM resveratrol did not affect the degree of apoptosis (p = 0.89), but 100 microM resveratrol increased the degree of apoptosis (p = 0.003). Both 10 and 100 microM resveratrol did not affect the degree of necrosis (p = 0.74, 0.33).
CONCLUSIONS
Resveratrol decreased cellular survival of cultured HTFBs and induced apoptosis. Thus, resveratrol may exert antiproliferative effects on HTFBs.
Keyword
Figure
Reference
-
References
1. Addicks EM, Quigley HA, Green WR, Robin AL. Histologic char-acteristics of filtering blebs. Arch Ophthalmol. 1983; 101:795–8.2. Skuta GL, Parrish RK. 2nd. Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987; 32:149–70.
Article3. Bindlish R, Condon GP, Schlosser JD. . Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002; 109:1336–41. discussion 1341-2.4. Migdal C, Hitchings R. Morbidity following prolonged post-operative hypotony after trabeculectomy. Ophthalmic Surg. 1988; 19:865–7.
Article5. Zacharia PT, Deppermann SR, Schuman JS. Ocular hypotony after trabeculectomy with mitomycin C. Am J Ophthalmol. 1993; 116:314–26.
Article6. Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003; 110:1185–91.
Article7. Lama PJ, Fechtner RD. Antifibrotics and wound healing in glauco-ma surgery. Surv Ophthalmol. 2003; 48:314–46.
Article8. Kim SH, Kim JW. Comparison of the effects between bevacizumab and mitomycin C on the survival of fibroblasts. J Korean Ophthalmol Soc. 2011; 52:345–9.
Article9. Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003; 17:1975–85.
Article10. Subramanian L, Youssef S, Bhattacharya S. . Resveratrol: chal-lenges in translation to the clinic-a critical discussion. Clin Cancer Res. 2010; 16:5942–8.11. Yu W, Fu YC, Wang W. Cellular and molecular effects of resvera-trol in health and disease. J Cell Biochem. 2012; 113:752–9.
Article12. Kim WT, Suh ES. Retinal protective effects of resveratrol via mod-ulation of nitric oxide synthase on oxygen-induced retinopathy. Korean J Ophthalmol. 2010; 24:108–18.
Article13. Nagaoka T, Hein TW, Yoshida A, Kuo L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: role of nitric oxide and potassium channels. Invest Ophthalmol Vis Sci. 2007; 48:4232–9.
Article14. Liu XQ, Wu BJ, Pan WH. . Resveratrol mitigates rat retinal is-chemic injury: the roles of matrix metalloproteinase-9, inducible nitric oxide, and heme oxygenase-1. J Ocul Pharmacol Ther. 2013; 29:33–40.
Article15. Liu Q, Ju WK, Crowston JG. . Oxidative stress is an early event in hydrostatic pressure induced retinal ganglion cell damage. Invest Ophthalmol Vis Sci. 2007; 48:4580–9.16. Luna C, Li G, Liton PB. . Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in tra-becular meshwork cells. Food Chem Toxicol. 2009; 47:198–204.
Article17. Poussier B, Cordova AC, Becquemin JP, Sumpio BE. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J Vasc Surg. 2005; 42:1190–7.
Article18. Sgambato A, Ardito R, Faraglia B. . Resveratrol, a natural phe-nolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat Res. 2001; 496:171–80.
Article19. Garcia P, Schmiedlin-Ren P, Mathias JS. . Resveratrol causes cell cycle arrest, decreased collagen synthesis, and apoptosis in rat intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2012; 302:G326–35.
Article20. Lee ES, Shin MO, Yoon S, Moon JO. Resveratrol inhibits dime-thylnitrosamine-induced hepatic fibrosis in rats. Arch Pharm Res. 2010; 33:925–32.
Article21. Li J, Qu X, Ricardo SD. . Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010; 177:1065–71.22. Mosmann T. Rapid colorimetric assay for cellular growth and sur-vival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article23. Green LC, Wagner DA, Glogowski J. . Analysis of nitrate, ni-trite and [15N]nitrate in biologic fluids. Anal Biochem. 1982; 126:131–8.24. Holian O, Wahid S, Atten MJ, Attar BM. Inhibition of gastric can-cer cell proliferation by resveratrol: role of nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2002; 282:G809–16.
Article25. Oktema G, Uysala A, Oral O. . Resveratrol attenuates doxor-ubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp Toxicol Pathol. 2012; 64:471–9.26. Dimmeler S, Zeiher AM. Nitric oxide and apoptosis: another para-digm for the double-edged role of nitric oxide. Nitric Oxide. 1997; 1:275–81.
Article27. Kröncke KD, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotox-icity versus cytoprotection- how, why, when, and where? Nitric Oxide. 1997; 1:107–20.28. Kim JW, Kim SK, Song IH, Kim IT. Mitomycin C-induced apopto-sis in cultured human Tenon’s capsule fibroblasts. Korean J Ophthalmol. 1999; 13:7–15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Valproic Acid on the Survival of Human Tennon's Capsule Fibroblasts
- Mitomycin C-induced apoptosis in cultured human Tenon's capsule fibroblasts
- Comparison of the Effects Between Bevacizumab and Mitomycin C on the Survival of Fibroblasts
- Effect of Amniotic Membrane Extract on the Tenon's Capsule Fibroblasts
- Effect of Gelrite on the Proliferation of Cultured Human Tenon's Capsule Fibroblasts