Korean J Obstet Gynecol.
2011 Apr;54(4):175-183. 10.5468/KJOG.2011.54.4.175.
The effect of cisplatin on endoplasmic reticulum stress of human cervical cancer cells
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea. vaginakim@hanmail.net
- 2Department of Radiology, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea.
- 3Department of Microbiology, Vestibulocochlear Research Center, Wonkwang University School of Medicine, Iksan, Korea.
- KMID: 2013231
- DOI: http://doi.org/10.5468/KJOG.2011.54.4.175
Abstract
OBJECTIVE
Cis-diamminedichloroplatinum (cisplatin) is a widely used chemotherapeutic agent. A number of evidences in cytotoxic mechanism of cisplatin, including perturbation of redox status, increase in lipid peroxydation, formation of DNA adduct, have been suggested. The author hypothesized that cisplatin would mediate apoptosis via endoplasmic reticulum (ER) stress in human cervical cancer cell.
METHODS
Human cervical cancer cell line (Hela cells) were treated with cisplatin and then ER stress-related response were performed using western blot, Flow cytometry and fluorescence analysis.
RESULTS
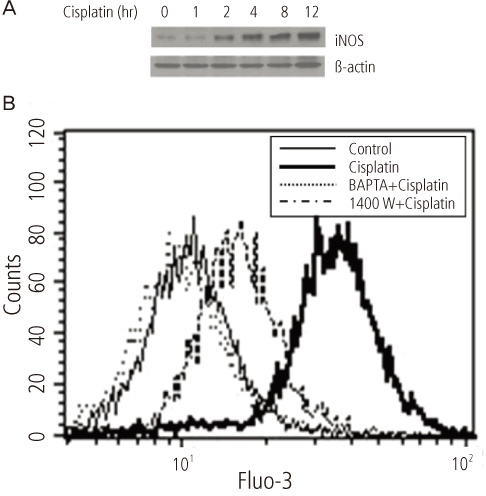
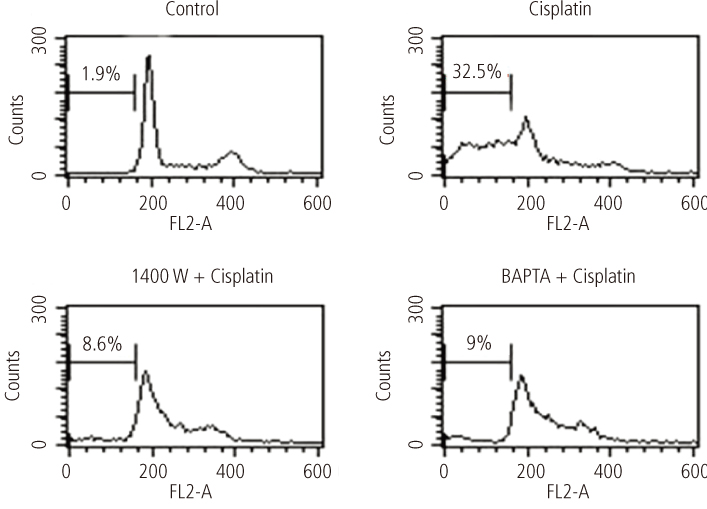
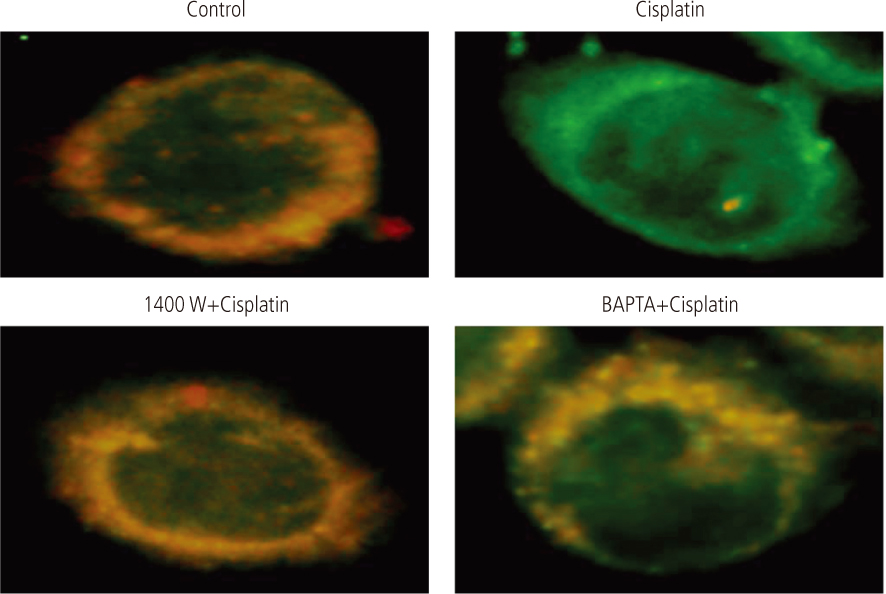
After addition of cisplatin to Hela cells, the author observed an expression of ER stress response genes through a gradual increase of nitric oxide and cytosolic Ca2+ concentration. Cisplatin-induced apoptosis can be inhibited by the inducible nitric oxide synthase inhibitor, 1400 W, and intracellular Ca2+ chelator, 1, 2-bis-(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetra-(acetoxymethyl) ester (BAPTA-AM). These inhibitors also reduced mitochondrial apoptotic signals, such as mitochondrion membrane potential disruption, cytochrome c release and eventually reduced the death of Hela cells.
CONCLUSION
Taken together, ER would seem to contribute to cisplatin-induced apoptosis via both the early release of Ca2+ and the late amplification of mitochondria-mediated apoptotic signals.
MeSH Terms
-
Apoptosis
Blotting, Western
Cell Line
Cisplatin
Cytochromes c
Cytosol
DNA
Endoplasmic Reticulum
Endoplasmic Reticulum Stress
Flow Cytometry
Fluorescence
HeLa Cells
Humans
Imines
Membrane Potentials
Mitochondria
Nitric Oxide
Nitric Oxide Synthase Type II
Oxidation-Reduction
Uterine Cervical Neoplasms
Cisplatin
Cytochromes c
DNA
Imines
Nitric Oxide
Nitric Oxide Synthase Type II
Figure
Reference
-
1. Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999. 13:1211–1233.2. Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000. 101:451–454.3. Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem. 2007. 282:31675–31687.4. Bobrovnikova-Marjon E, Diehl JA. Coping with stress: ATF6alpha takes the stage. Dev Cell. 2007. 13:322–324.5. Gass JN, Jiang HY, Wek RC, Brewer JW. The unfolded protein response of B-lymphocytes: PERK-independent development of antibody-secreting cells. Mol Immunol. 2008. 45:1035–1043.6. Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshoda H, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007. 13:365–376.7. Endo H, Murata K, Mukai M, Ishikawa O, Inoue M. Activation of insulin-like growth factor signaling induces apoptotic cell death under prolonged hypoxia by enhancing endoplasmic reticulum stress response. Cancer Res. 2007. 67:8095–8103.8. Henshall DC, Murphy BM. Modulators of neuronal cell death in epilepsy. Curr Opin Pharmacol. 2008. 8:75–81.9. Fram RJ. Cisplatin and platinum analogues: Recent advances. Curr Opin Oncol. 1992. 4:1073–1079.10. Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002. 7:335–345.11. Harding HP, Zhang Y, Ron D. PERK is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000. 5:897–904.12. Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998. 17:5708–5717.13. Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998. 273:33741–33749.14. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004. 11:381–389.15. Chami M, Oulès B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Bréchot P. Role of SERCA1 Truncated Isoform in the Proapoptotic Calcium Transfer from ER to Mitochondria during ER Stress. Mol Cell. 2008. 32:641–651.16. Qi X, Vallentin A, Churchill E, Mochly-Rosen D. deltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart. J Mol Cell Cardiol. 2007. 43:420–428.17. Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, et al. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004. 11:403–415.18. Florea SM, Blatter LA. The effect of oxidative stress on Ca2+ release and capacitative Ca2+ entry in vascular endothelial cells. Cell Calcium. 2008. 43:405–415.19. Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, et al. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J Biol Chem. 2005. 280:33917–33925.20. Feghali JG, Liu W, Van De Water TR. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope. 2001. 111:1147–1155.21. Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci. 2000. 57:1229–1235.22. Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001. 478:23–43.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoplasmic Reticulum Stress and Diabetes
- Cynaropicrin Induces Reactive Oxygen Species-Dependent Paraptosis-Like Cell Death in Human Liver Cancer Cells
- New Insights into the Role of Endoplasmic Reticulum Stress in Breast Cancer Metastasis
- Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells
- Endoplasmic Reticulum Stress Responses and Apoptosis