Dipeptides Inhibit Melanin Synthesis in Mel-Ab Cells through Down-Regulation of Tyrosinase
- Affiliations
-
- 1Department of Biochemistry, Chung-Ang University College of Medicine, Seoul 156-756, Korea. ds_kim@cau.ac.kr
- 2Department of Dermatology, Seoul National University College of Medicine, Seoul 110-744, Korea.
- 3Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 2011164
- DOI: http://doi.org/10.4196/kjpp.2012.16.4.287
Abstract
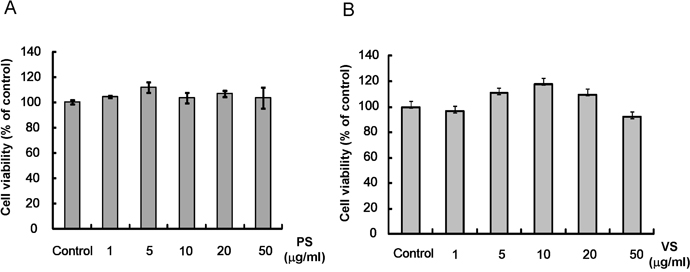
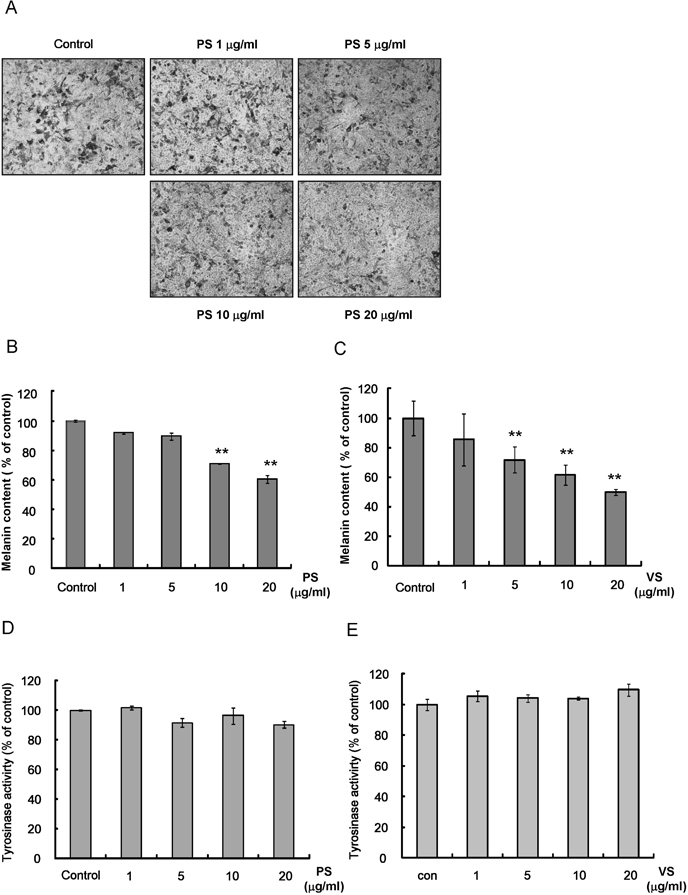
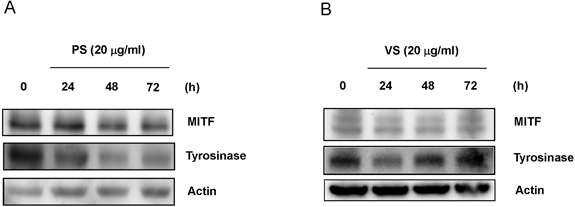
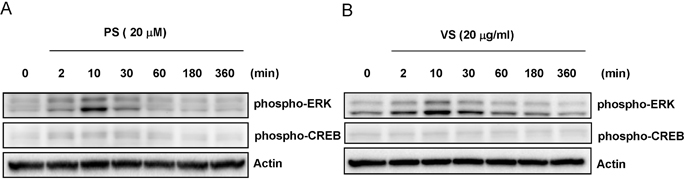
- This study investigated the effects of proline-serine (PS) and valine-serine (VS) dipeptides on melanogenesis in Mel-Ab cells. Proline-serine and VS significantly inhibited melanin synthesis in a concentration-dependent manner, though neither dipeptide directly inhibited tyrosinase activity in a cell-free system. Both PS and VS down-regulated the expression of microphthalmia-associated transcription factor (MITF) and tyrosinase. In a follow-up study also described here, the effects of these dipeptides on melanogenesis-related signal transduction were quantified. Specifically, PS and VS induced ERK phosphorylation, though they had no effect on phosphorylation of the cAMP response element binding protein (CREB). These data suggest that PS and VS inhibit melanogenesis through ERK phosphorylation and subsequent down-regulation of MITF and tyrosinase. Properties of these dipeptides are compatible with application as skin-whitening agents.
Keyword
MeSH Terms
-
Cell-Free System
Cyclic AMP Response Element-Binding Protein
Dipeptides
Down-Regulation
Follow-Up Studies
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Phosphorylation
Signal Transduction
Cyclic AMP Response Element-Binding Protein
Dipeptides
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Figure
Cited by 3 articles
-
KHG26792 Inhibits Melanin Synthesis in Mel-Ab Cells and a Skin Equivalent Model
Hailan Li, Jandi Kim, Hoh-Gyu Hahn, Jun Yun, Hyo-Soon Jeong, Hye-Young Yun, Kwang Jin Baek, Nyoun Soo Kwon, Young Sil Min, Kyoung-Chan Park, Dong-Seok Kim
Korean J Physiol Pharmacol. 2014;18(3):249-254. doi: 10.4196/kjpp.2014.18.3.249.Scopoletin from
Cirsium setidens Increases Melanin Synthesis via CREB Phosphorylation in B16F10 Cells
Mi-Ja Ahn, Sun-Jung Hur, Eun-Hyun Kim, Seung Hoon Lee, Jun Seob Shin, Myo-Kyoung Kim, James A. Uchizono, Wan-Kyunn Whang, Dong-Seok Kim
Korean J Physiol Pharmacol. 2014;18(4):307-311. doi: 10.4196/kjpp.2014.18.4.307.ERK Activation by Fucoidan Leads to Inhibition of Melanogenesis in Mel-Ab Cells
Yu Seok Song, Marie Carmel Balcos, Hye-Young Yun, Kwang Jin Baek, Nyoun Soo Kwon, Myo-Kyoung Kim, Dong-Seok Kim
Korean J Physiol Pharmacol. 2015;19(1):29-34. doi: 10.4196/kjpp.2015.19.1.29.
Reference
-
1. del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996. 381:165–168.2. Ando H, Niki Y, Yoshida M, Ito M, Akiyama K, Kim JH, Yoon TJ, Matsui MS, Yarosh DB, Ichihashi M. Involvement of pigment globules containing multiple melanosomes in the transfer of melanosomes from melanocytes to keratinocytes. Cell Logist. 2011. 1:12–20.3. Schwahn DJ, Xu W, Herrin AB, Bales ES, Medrano EE. Tyrosine levels regulate the melanogenic response to alpha-melanocyte-stimulating hormone in human melanocytes: implications for pigmentation and proliferation. Pigment Cell Res. 2001. 14:32–39.4. Goding CR. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000. 14:1712–1728.5. Ohguchi K, Akao Y, Nozawa Y. Involvement of calpain in melanogenesis of mouse B16 melanoma cells. Mol Cell Biochem. 2005. 275:103–107.6. Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998. 391:298–301.7. Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000. 13:60–69.8. Kim SY, Na JI, Park SJ, Choi HR, Kim DS, Park KC. Novel tri-peptides with hypopigmenting activity. J Dermatol Sci. 2012. 65:68–69.9. Bardan A, Nizet V, Gallo RL. Antimicrobial peptides and the skin. Expert Opin Biol Ther. 2004. 4:543–549.10. Kim H, Lee BJ, Lee MH, Hong SG, Ryu PD. Mechanisms of selective antimicrobial activity of gaegurin 4. Korean J Physiol Pharmacol. 2009. 13:39–47.11. Zhang L, Falla TJ. Cosmeceuticals and peptides. Clin Dermatol. 2009. 27:485–494.12. Noh JM, Kwak SY, Kim DH, Lee YS. Kojic acid-tripeptide amide as a new tyrosinase inhibitor. Biopolymers. 2007. 88:300–307.13. Abdel-Malek ZA, Kadekaro AL, Kavanagh RJ, Todorovic A, Koikov LN, McNulty JC, Jackson PJ, Millhauser GL, Schwemberger S, Babcock G, Haskell-Luevano C, Knittel JJ. Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. FASEB J. 2006. 20:1561–1563.14. Abu Ubeid A, Zhao L, Wang Y, Hantash BM. Short-sequence oligopeptides with inhibitory activity against mushroom and human tyrosinase. J Invest Dermatol. 2009. 129:2242–2249.15. Schurink M, Van BW, Wichers HJ, Boeriu CG. Novel peptides with tyrosinase inhibitory activity. Peptides. 2007. 28:485–495.16. Ishikawa M, Kawase I, Ishii F. Combination of amino acids reduces pigmentation in B16F0 melanoma cells. Biol Pharm Bull. 2007. 30:677–681.17. Dooley TP, Gadwood RC, Kilgore K, Thomasco LM. Development of an in vitro primary screen for skin depigmentation and antimelanoma agents. Skin Pharmacol. 1994. 7:188–200.18. Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res. 1998. 11:275–282.19. Kim DS, Park SH, Jeong YM, Kwon SB, Miller AJ, Fisher DE, Park KC. Sphingosine-1-phosphate decreases melanin synthesis via microphthalmia-associated transcription factor phosphorylation through the S1P3 receptor subtype. J Pharm Pharmacol. 2011. 63:409–416.20. Buscà R, Bertolotto C, Ortonne JP, Ballotti R. Inhibition of the phosphatidylinositol 3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell differentiation. J Biol Chem. 1996. 271:31824–31830.21. Kondo T, Hearing VJ. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol. 2011. 6:97–108.22. Kang YA, Na JI, Choi HR, Choi JW, Kang HY, Park KC. Novel anti-inflammatory peptides as cosmeceutical peptides. Peptides. 2011. 32:2134–2136.23. Nitta A, Nishioka H, Fukumitsu H, Furukawa Y, Sugiura H, Shen L, Furukawa S. Hydrophobic dipeptide Leu-Ile protects against neuronal death by inducing brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis. J Neurosci Res. 2004. 78:250–258.24. Anisimov VN, Khavinson VK, Morozov VG. Immunomodulatory synthetic dipeptide L-Glu-L-Trp slows down aging and inhibits spontaneous carcinogenesis in rats. Biogerontology. 2000. 1:55–59.25. Shibahara S, Takeda K, Yasumoto K, Udono T, Watanabe K, Saito H, Takahashi K. Microphthalmia-associated transcription factor (MITF): multiplicity in structure, function, and regulation. J Investig Dermatol Symp Proc. 2001. 6:99–104.26. Kim DS, Hwang ES, Lee JE, Kim SY, Kwon SB, Park KC. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J Cell Sci. 2003. 116:1699–1706.27. Kim H, Choi J, Cho JK, Kim SY, Lee YS. Solid-phase synthesis of kojic acid-tripeptides and their tyrosinase inhibitory activity, storage stability, and toxicity. Bioorg Med Chem Lett. 2004. 14:2843–2846.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical and Electron Microscopic Study on the Depigmentation Effect of Melanoston
- ERK Activation by Fucoidan Leads to Inhibition of Melanogenesis in Mel-Ab Cells
- The effects of green tea (Camellia sinensis) flower extracton melanin synthesis in B16-F10 melanoma cells
- KHG26792 Inhibits Melanin Synthesis in Mel-Ab Cells and a Skin Equivalent Model
- Effect of Adenosine on Melanogenesis in B16 Cells and Zebrafish