Korean J Physiol Pharmacol.
2014 Jun;18(3):249-254. 10.4196/kjpp.2014.18.3.249.
KHG26792 Inhibits Melanin Synthesis in Mel-Ab Cells and a Skin Equivalent Model
- Affiliations
-
- 1Department of Biochemistry, Chung-Ang University College of Medicine, Seoul 156-756, Korea. ds_kim@cau.ac.kr
- 2Organic Chemistry Laboratory, Korea Institute of Science & Technology, Seoul 136-791, Korea.
- 3Department of Herb Industry, Jungwon University, Goesan 367-805, Korea.
- 4Department of Dermatology, Seoul National University Bundang Hospital, Seongnam 463-707, Korea.
- KMID: 2285517
- DOI: http://doi.org/10.4196/kjpp.2014.18.3.249
Abstract
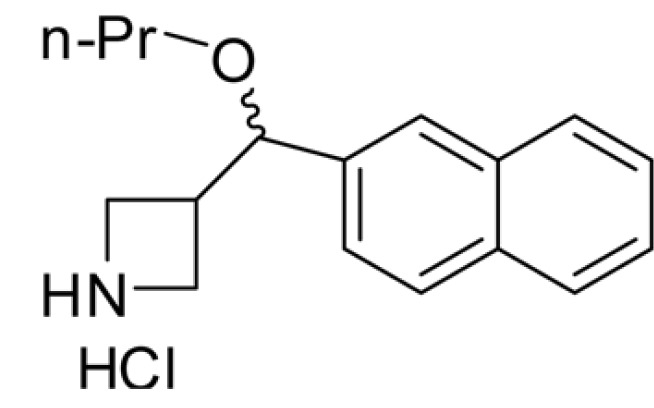
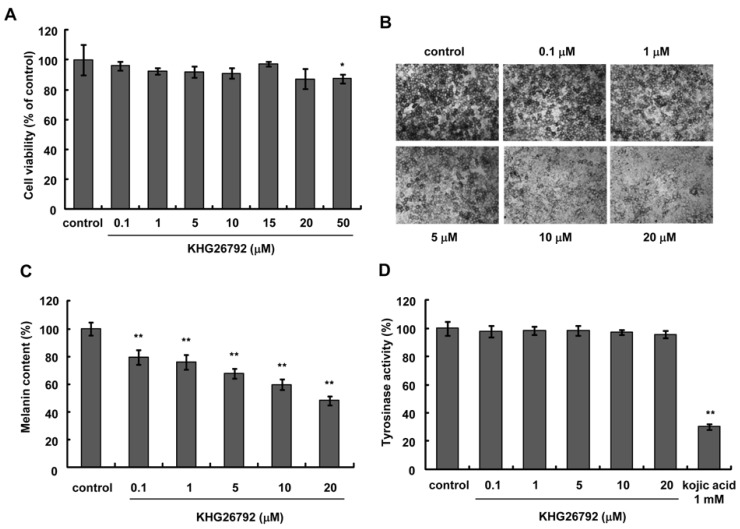
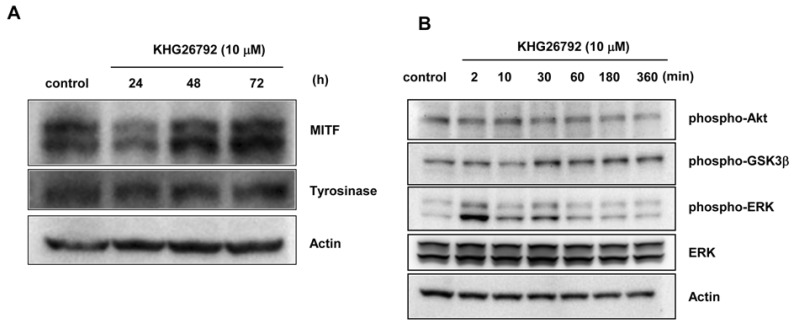
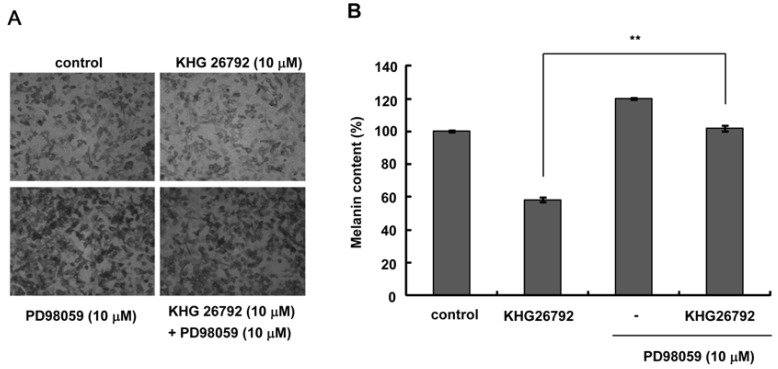
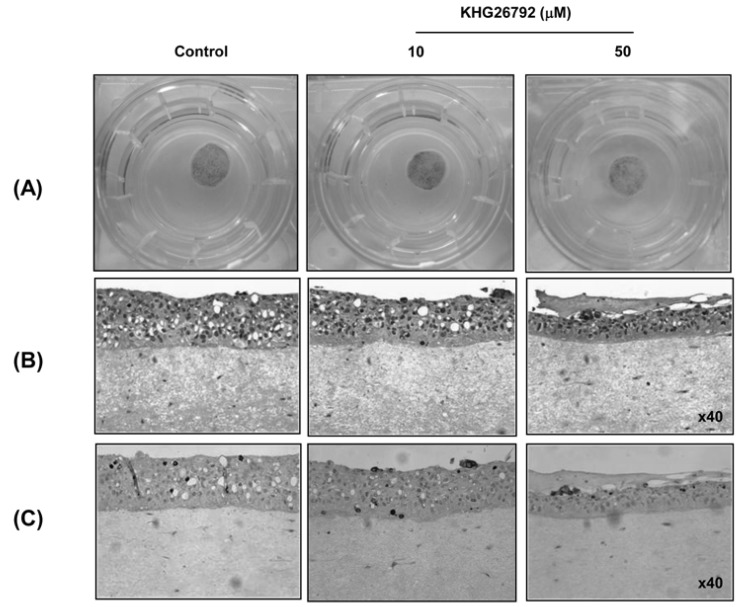
- The purpose of this study is to characterize the effects of KHG26792 (3-(naphthalen-2-yl(propoxy) methyl)azetidine hydrochloride), a potential skin whitening agent, on melanin synthesis and identify the underlying mechanism of action. Our data showed that KHG26792 significantly reduced melanin synthesis in a dose-dependent manner. Additionally, KHG26792 downregulated microphthalmia-associated transcription factor (MITF) and tyrosinase, the rate-limiting enzyme in melanogenesis, although tyrosinase was not inhibited directly. KHG26792 activated extracellular signal-regulated kinase (ERK), whereas an ERK pathway inhibitor, PD98059, rescued KHG26792-induced hypopigmentation. These results suggest that KHG26792 decreases melanin production via ERK activation. Moreover, the hypopigmentary effects of KHG26792 were confirmed in a pigmented skin equivalent model using Cervi cornus Colla (deer antler glue), in which the color of the pigmented artificial skin became lighter after treatment with KHG26792. In summary, our findings suggest that KHG26792 is a novel skin whitening agent.
Keyword
MeSH Terms
-
Animals
Antlers
Cornus
Hypopigmentation
MAP Kinase Signaling System
Melanins*
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Phosphotransferases
Skin Lightening Preparations
Skin*
Skin, Artificial
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Phosphotransferases
Skin Lightening Preparations
Figure
Cited by 1 articles
-
ERK Activation by Fucoidan Leads to Inhibition of Melanogenesis in Mel-Ab Cells
Yu Seok Song, Marie Carmel Balcos, Hye-Young Yun, Kwang Jin Baek, Nyoun Soo Kwon, Myo-Kyoung Kim, Dong-Seok Kim
Korean J Physiol Pharmacol. 2015;19(1):29-34. doi: 10.4196/kjpp.2015.19.1.29.
Reference
-
1. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009; 9:679–691. PMID: 19763149.
Article2. Cho YS, Lee KH, Park JW. Pyrithione-zinc Prevents UVB-induced Epidermal Hyperplasia by Inducing HIF-1alpha. Korean J Physiol Pharmacol. 2010; 14:91–97. PMID: 20473380.3. Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007; 282:27557–27561. PMID: 17635904.
Article4. Aoki Y, Tanigawa T, Abe H, Fujiwara Y. Melanogenesis inhibition by an oolong tea extract in b16 mouse melanoma cells and UV-induced skin pigmentation in brownish guinea pigs. Biosci Biotechnol Biochem. 2007; 71:1879–1885. PMID: 17690471.
Article5. Kim SY, Na JI, Park SJ, Choi HR, Kim DS, Park KC. Novel tri-peptides with hypopigmenting activity. J Dermatol Sci. 2012; 65:68–69. PMID: 22154815.
Article6. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007; 21:976–994. PMID: 17242160.
Article7. Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991; 5:2902–2909. PMID: 1752358.
Article8. Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000; 13:60–69. PMID: 10841026.9. Kim DS, Park SH, Kwon SB, Park ES, Huh CH, Youn SW, Park KC. Sphingosylphosphorylcholine-induced ERK activation inhibits melanin synthesis in human melanocytes. Pigment Cell Res. 2006; 19:146–153. PMID: 16524430.
Article10. Kim DS, Hwang ES, Lee JE, Kim SY, Kwon SB, Park KC. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J Cell Sci. 2003; 116:1699–1706. PMID: 12665551.
Article11. Faller C, Bracher M, Dami N, Roguet R. Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicol In Vitro. 2002; 16:557–572. PMID: 12206823.
Article12. Kim J, Jeong HS, Li H, Baek KJ, Kwon NS, Yun HY, Choi HR, Park KC, Kim DS. Effects of Cervi cornus Colla (deer antler glue) in the reconstruction of a skin equivalent model. Arch Dermatol Res. 2013; 305:85–89. PMID: 23011660.
Article13. Han Y, Han M, Shin D, Song C, Hahn HG. Exploration of novel 3-substituted azetidine derivatives as triple reuptake inhibitors. J Med Chem. 2012; 55:8188–8192. PMID: 22938049.
Article14. Dooley TP, Gadwood RC, Kilgore K, Thomasco LM. Development of an in vitro primary screen for skin depigmentation and antimelanoma agents. Skin Pharmacol. 1994; 7:188–200. PMID: 8024800.
Article15. Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988; 106:761–771. PMID: 2450098.
Article16. Tsuboi T, Kondoh H, Hiratsuka J, Mishima Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment Cell Res. 1998; 11:275–282. PMID: 9877098.
Article17. Auger FA, López Valle CA, Guignard R, Tremblay N, Noël B, Goulet F, Germain L. Skin equivalent produced with human collagen. In Vitro Cell Dev Biol Anim. 1995; 31:432–439. PMID: 8589886.
Article18. del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996; 381:165–168. PMID: 8601447.
Article19. Kim SY, Hahn HG, Nam KD, Park KC, Yun HY, Baek KJ, Kwon NS, Kim DS. A derivative of 2-aminothiazole inhibits melanogenesis in B16 mouse melanoma cells via glycogen synthase kinase 3β phosphorylation. J Pharm Pharmacol. 2011; 63:1031–1036. PMID: 21718286.
Article20. Lee HE, Kim EH, Choi HR, Sohn UD, Yun HY, Baek KJ, Kwon NS, Park KC, Kim DS. Dipeptides Inhibit Melanin Synthesis in Mel-Ab Cells through Down-Regulation of Tyrosinase. Korean J Physiol Pharmacol. 2012; 16:287–291. PMID: 22915995.
Article21. Noh JM, Kwak SY, Kim DH, Lee YS. Kojic acid-tripeptide amide as a new tyrosinase inhibitor. Biopolymers. 2007; 88:300–307. PMID: 17211869.
Article22. Kim EH, Jeong HS, Yun HY, Baek KJ, Kwon NS, Park KC, Kim DS. Geranylgeranylacetone inhibits melanin synthesis via ERK activation in Mel-Ab cells. Life Sci. 2013; 93:226–232. PMID: 23792203.
Article23. Poumay Y, Coquette A. Modelling the human epidermis in vitro: tools for basic and applied research. Arch Dermatol Res. 2007; 298:361–369. PMID: 17072628.
Article24. Ponec M, Boelsma E, Weerheim A, Mulder A, Bouwstra J, Mommaas M. Lipid and ultrastructural characterization of reconstructed skin models. Int J Pharm. 2000; 203:211–225. PMID: 10967443.
Article25. Grabbe J, Welker P, Dippel E, Czarnetzki BM. Stem cell factor, a novel cutaneous growth factor for mast cells and melanocytes. Arch Dermatol Res. 1994; 287:78–84. PMID: 7537033.
Article26. Yada Y, Higuchi K, Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991; 266:18352–18357. PMID: 1917960.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Involvement of Transglutaminase-2 in alpha-MSH-Induced Melanogenesis in SK-MEL-2 Human Melanoma Cells
- ERK Activation by Fucoidan Leads to Inhibition of Melanogenesis in Mel-Ab Cells
- Dipeptides Inhibit Melanin Synthesis in Mel-Ab Cells through Down-Regulation of Tyrosinase
- The effects of green tea (Camellia sinensis) flower extracton melanin synthesis in B16-F10 melanoma cells
- Pigmentation Effect of Rice Bran Extract in Hair Follicle-Like Tissue and Organ Culture Models