Chonnam Med J.
2008 Apr;44(1):1-9. 10.4068/cmj.2008.44.1.1.
Particulate Air Pollutants and Airway Inflammation
- Affiliations
-
- 1Asthma and Allergy Research Group, Division of Allergy and Respiratory Diseases, Soonchunhyang University Hospital, Bucheon, Korea. jas877@schbc.ac.kr
- 2Department of Allergy, Chonnam National University Medical School and the Research Institute of Medical Sciences, Gwangju, Korea.
- KMID: 1973383
- DOI: http://doi.org/10.4068/cmj.2008.44.1.1
Abstract
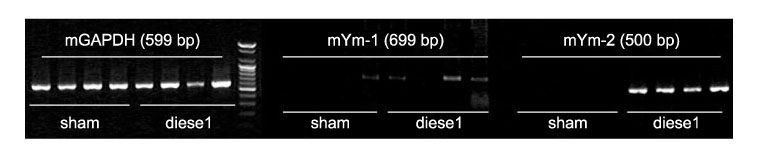
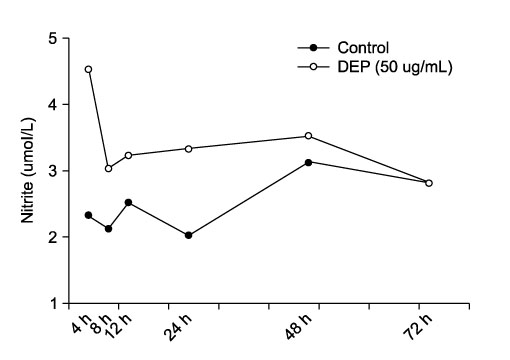
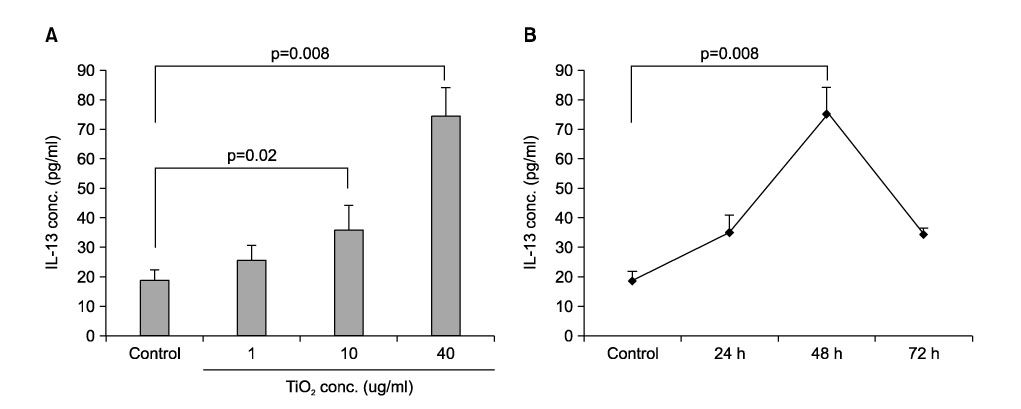
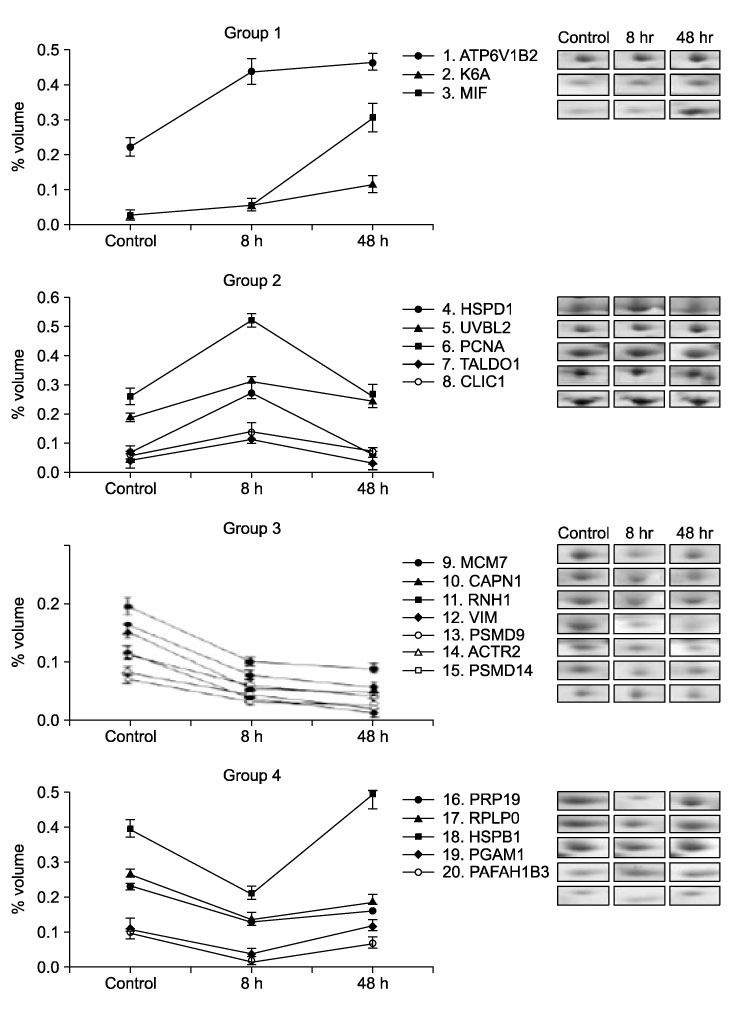
- Particulate air pollutants emanating from traffic and various industries are related to allergic airway disorders including asthma. Particulate air pollutants inhalation directly induces lung inflammation to allergens or respiratory viral infection as an adjuvant by macrophages and epithelial cells. Inhalation of particulate air pollutants aggravates respiratory symptoms in patients with chronic airway diseases, but the mechanisms underlying this response remain poorly understood. Diesel exhaust particles induced airway hyperresponsiveness and Ym mRNA expression via a Th2 cell-biased response, which synthesized by activated macrophages are homologous to chitinase and have chitinase activity. Alveolar macrophages play an important role in particles-induced airway and lung inflammation via direct production of IL-13. Treatment of epithelial cells with bovine serum albumin coated titanium dioxide particles altered 20 protein spots on the two-dimensional gel, and these were then analyzed by nano-LC-MS/MS. These proteins included defense-related, cell-activating, and cytoskeletal proteins implicated in the response to oxidative stress. TiO2 treatment was found to increase the amount of mRNA for macrophage migration-inhibitory factor (MIF). MIF was expressed primarily in epithelium and was elevated in lung tissues and bronchoalveolar lavage fluids of TiO2-treated rats as compared with sham treated rats. Carbon black and diesel exhaust particles also induced expression of MIF protein in the epithelial cells. We attempt to offer insight into how particles may influence airway inflammation.
Keyword
MeSH Terms
-
Air Pollution
Allergens
Animals
Asthma
Bronchoalveolar Lavage Fluid
Chitinase
Cytoskeletal Proteins
Epithelial Cells
Epithelium
Humans
Inflammation
Inhalation
Interleukin-13
Lung
Macrophages
Macrophages, Alveolar
Oxidative Stress
Particulate Matter
Pneumonia
Proteins
Rats
RNA, Messenger
Salicylamides
Serum Albumin, Bovine
Soot
Titanium
Vehicle Emissions
Allergens
Chitinase
Cytoskeletal Proteins
Interleukin-13
Particulate Matter
Proteins
RNA, Messenger
Salicylamides
Serum Albumin, Bovine
Soot
Titanium
Vehicle Emissions
Figure
Reference
-
1. McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007; 357:2348–2358.
Article2. Dockery DW, Pope CA 3rd, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993; 329:1753–1759.
Article3. Pope CA 3rd, Kanner RE. Acute effects of PM10 pollution on pulmonary function of smokers with mild to moderate chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993; 147:1336–1340.4. Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis. 1993; 147:826–831.
Article5. Schäfer T, Ring J. Epidemiology of allergic diseases. Allergy. 1997; 52 38 Suppl:S14–S22.6. Pagan I, Costa DL, McGee JK, Richards JH, Dye JA. Metal mimic airway epithelial injury induced by in vitro exposure to Utah Valley ambient particulate matter extracts. J Toxicol Environ Health A. 2003; 66:1087–1112.7. Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997; 158:2406–2413.8. Fujieda S, Diaz-Sanchez D, Saxon A. Combined nasal challenge with diesel exhaust particles and allergen induces in vivo IgE isotype switching. Am J Respir Cell Mol Biol. 1998; 19:507–512.9. Fahy O, Sénéchal S, Péne J, Scherpereel A, Lassalle P, Tonnel A. Diesel exposure favors Th2 cell recruitment by mononuclear cells and alveolar macrophages from allergic patients by differentially regulating macrophage-derived chemokine and IFN-gamma-induced protein-10 production. J Immunol. 2002; 168:5912–5919.10. Hamilton RF Jr, Holian A, Morandi MT. A comparison of asbestos and urban particulate matter in the in vitro modification of human alveolar macrophage antigen-presenting cell function. Exp Lung Res. 2004; 30:147–162.
Article11. Peden DB. Pollutants and asthma: role of air toxics. Environ Health Perspect. 2002; 110 Suppl 4:S565–S568.
Article12. Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996; 51:1216–1222.
Article13. Ghio AJ, Devlin RB. Inflammatory lung injury after bronchial instillation of air pollution particles. Am J Respir Crit Care Med. 2001; 164:704–708.
Article14. Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995; 345:176–178.
Article15. Zelikoff JT, Chen LC, Cohen MD, Fang K, Gordon T, Li Y, et al. Effects of inhaled ambient particulate matter on pulmonary antimicrobial immune defense. Inhal Toxicol. 2003; 15:131–150.
Article16. Becker S, Soukup JM, Gilmour MI, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996; 141:637–648.
Article17. Fujii T, Hayashi S, Hogg JC, Vincent R, Van Eeden SF. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001; 25:265–271.
Article18. Brody AR, Bonner JC, Overby LH, Badgett A, Kalter V, Kumar RK, et al. Interstitial pulmonary macrophages produce platelet-derived growth factor that stimulates rat lung fibroblast proliferation in vitro. J Leukoc Biol. 1992; 51:640–648.
Article19. Lindroos PM, Coin PG, Badgett A, Morgan DL, Bonner JC. Alveolar macrophages stimulated with titanium dioxide, chrysotile asbestos, and residual oil fly ash upregulate the PDGF receptor-alpha on lung fibroblasts through an IL-1beta-dependent mechanism. Am J Respir Cell Mol Biol. 1997; 16:283–292.
Article20. Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003; 111:455–460.
Article21. Templeton DM. Titanium. In: Seiler HG, Siegel A, Siegel H, editors. Handbook on metals in clinical and analytic chemistry. New York: Marcel Dekker; 1994. pp. 627-630.22. Garabrant DH, Fine LJ, Oliver C, Bernstein L, Peters JM. Abnormalities of pulmonary function and pleural disease among titanium metal production workers. Scand J Work Environ Health. 1987; 13:47–51.
Article23. Ahn MH, Kang CM, Park CS, Park SJ, Rhim T, Yoon PO, et al. Titanium dioxide particle-induced goblet cell hyperplasia: association with mast cells and IL-13. Respir Res. 2005; 6:34.
Article24. Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005; 33:290–296.
Article25. Schapira RM, Ghio AJ, Effros RM, Morrisey J, Almagro UA, Dawson CA, et al. Hydroxyl radical production and lung injury in the rat following silica or titanium dioxide instillation in vivo. Am J Respir Cell Mol Biol. 1995; 12:220–226.
Article26. Warheit DB, Hansen JF, Yuen IS, Kelly DP, Snajdr SI, Hartsky MA. Inhalation of high concentrations of low toxicity dusts in rats results in impaired pulmonary clearance mechanisms and persistent inflammation. Toxicol Appl Pharmacol. 1997; 145:10–22.
Article27. Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003; 278:50781–50790.
Article28. Blackford JA Jr, Jones W, Dey RD, Castranova V. Comparison of inducible nitric oxide synthase gene expression and lung inflammation following intratracheal instillation of silica, coal, carbonyl iron, or titanium dioxide in rats. J Toxicol Environ Health. 1997; 51:203–218.
Article29. Song HM, Jang AS, Ahn MH, Takizawa H, Lee SH, Kwon JH, et al. Ym1 and Ym2 expression in a mouse model exposed to diesel exhaust particles. Environ Toxicol. 2008; 23:110–116.
Article30. Cha MH, Rhim T, Kim KH, Jang AS, Paik YK, Park CS. Proteomic identification of macrophage migration-inhibitory factor upon exposure to TiO2 particles. Mol Cell Proteomics. 2007; 6:56–63.
Article31. Nicolai T. Air pollution and respiratory disease in children: what is the clinically relevant impact? Pediatr Pulmonol Suppl. 1999; 18:9–13.
Article32. Brunekreef B, Janssen AH, de Hartog J, Harssema H, Knape M, van Vliet P. Air pollution from truck traffic and lung function in children living near motoways. Epidemiology. 1997; 8:298–303.33. Koenig JQ. Air pollution and asthma. J Allergy Clin Immunol. 1999; 104:717–722.
Article34. Hirsch T, Weiland SK, von Mutius E, Safeca AF, Gräfe H, Csaplovics E, et al. Inner city air pollution and respiratory health and atopy in children. Eur Respir J. 1999; 14:669–677.
Article35. Ciccone G, Forastiere F, Agabiti N, Biggeri A, Bisanti L, Chellini E, et al. Road traffic and adverse respiratory effects in children. Occup Environ Med. 1998; 55:771–778.36. McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: the AHSMOG study. Environ Res. 1999; 80:110–121.
Article37. Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, et al. A study of twelve Southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999; 159:768–775.
Article38. Kreit JW, Gross KB, Moore TB, Lorenzen TJ, D'Arcy J, Eschenbacher WL. Ozone-induced changes in pulmonary function and bronchial responsiveness in asthmatics. J Appl Physiol. 1989; 66:217–222.
Article39. Folinsbee LJ. Does nitrogen dioxide exposure increase airways responsiveness. Toxicol Indust Health. 1992; 8:273–283.
Article40. Baldi I, Tessier JF, Kauffmann F, Jacqmin-Gadda H, Nejjari C, Salamon R. Prevalence of asthma and mean levels of air pollution: results from the French PAARC survey. Pollution Atomosphérique et Affections Respiratoires Chroniques. Eur Respir J. 1999; 14:132–138.
Article41. Gielen MH, van der Zee SC, van Wijnen JH, van Steen CJ, Brunekreef B. Acute effects of summer air pollution on respiratory health of asthmatic children. Am J Respir Crit Care Med. 1997; 155:2105–2108.
Article42. Jang AS, Yeum CH, Son MH. Epidemiologic evidence of a relationship between airway hyperresponsiveness and exposure to polluted air. Allergy. 2003; 58:585–588.
Article43. Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997; 156:766–775.
Article44. Guo L, Johnson RS, Schuh JC. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem. 2000; 275:8032–8037.
Article45. Sun YJ, Chang NC, Hung SI, Chang AC, Chou CC, Hsiao CD. The crystal structure of a novel mammalian lectin, Ym1, suggests a saccharide binding site. J Biol Chem. 2001; 276:17507–17514.
Article46. Jin HM, Copeland NG, Gilbert DJ, Jenkins NA, Kirkpatrick RB, Rosenberg M. Genetic characterization of the murine Ym1 gene and identification of a cluster of highly homologous genes. Genomics. 1998; 54:316–322.
Article47. Welch JS, Escoubet-Lozach L, Sykes DB, Liddiard K, Greaves DR, Glass CK. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J Biol Chem. 2002; 277:42821–42829.
Article48. Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004; 304:1678–1682.
Article49. Bierbaum S, Nickel R, Koch A, Lau S, Deichmann KA, Wahn U, et al. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am J Respir Crit Care Med. 2005; 172:1505–1509.
Article50. Finkelman FD, Urban JF Jr. The other side of the coin: the protective role of the TH2 cytokines. J Allergy Clin Immunol. 2001; 107:772–780.
Article51. Ward JM, Yoon M, Anver MR, Haines DC, Kudo G, Gonzalez FJ, et al. Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B6, 129 mice. Am J Pathol. 2001; 158:323–332.52. Moncada S, Palmer RM, Higgs EA. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989; 11:1709–1715.
Article53. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991; 143:109–142.54. Jang AS, Choi IS, Lee S, Seo JP, Yang SW, Park KO, et al. Nitric oxide metabolites in induced sputum: a marker of airway inflammation in asthmatic subjects. Clin Exp Allergy. 1999; 29:1136–1142.
Article55. Jang AS, Choi IS. Nitric oxide metabolites in patients with asthma: induced sputum versus blood. Respir Med. 1999; 93:912–918.
Article56. Jang AS, Choi IS, Lee JU, Park SW, Lee JH, Park CS. Changes in the expression of NO synthase isoforms after ozone: the effects of allergen exposure. Respir Res. 2004; 5:5.
Article