Tuberc Respir Dis.
2007 Apr;62(4):299-307. 10.4046/trd.2007.62.4.299.
Proinflammatory Effects of High Mobility Group B1 (HMGB1) Versus LPS and the Mechanism of IL-8 Promoter Stimulation by HMGB1
- Affiliations
-
- 1Department of Internal Medicine, ChungAng University College of Medicine, Seoul, Korea. jykimmd@cau.ac.kr
- KMID: 1970230
- DOI: http://doi.org/10.4046/trd.2007.62.4.299
Abstract
-
BACKGROUND: High mobility group box 1 (HMGB1) is a novel, late mediator of inflammation. This study compared the pro-inflammatory effects of LPS and HMGB1. The transcriptional factors that play an important role in mediating the HMGB1-induced stimulation of IL-8 were also evaluated.
METHODS
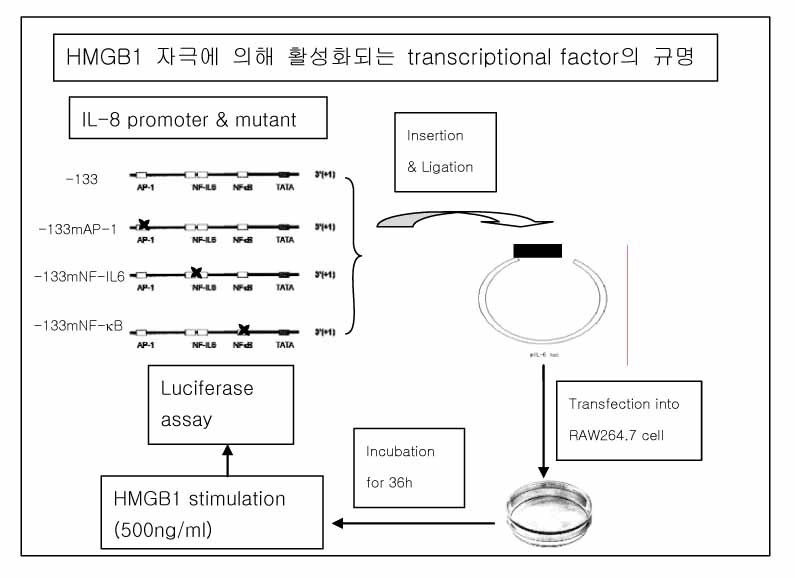
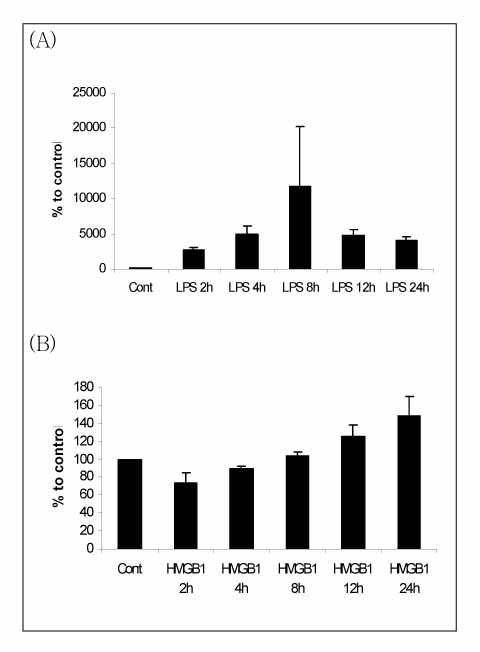
RAW264.7 cells were stimulated with either LPS (100 ng/ml) or HMGB1 (500 ng/ml). The TNF-alpha, MIP-2 and IL-1beta levels in the supernatant were evaluated by ELISA at 0, 2, 4, 8, 12 and 24h after stimulation. An acute lung injury was induced by an injection of LPS (5 mg/kg) or HMGB1 (2.5 mg/kg) into the peritoneum of the Balb/c mice. The lung cytokines and MPO activity were measured at 4h (for LPS) or 24h (for HMGB1) after the injection. The transcriptional factor binding sites for NF-IL6, NF-kappaB and AP-1 in the IL-8 promoter region were artificially mutated. Each mutant was ligated with pIL-6luc and transfected into the RAW264.7 cells. One hour after stimulation with HMGB1 (500 ng/ml), the cell lysate was analyzed for the luciferase activity.
RESULTS
The expression of MIP-2, which peaked at 8h with LPS stimulation, increased sequentially until 24h after HMGB1 stimulation. An intraperitoneal injection of HMGB1, which induced a minimal increased in IL-1beta expression, provoked the accumulation of neutrophils the lung. A mutation of AP-1 as well as NF-kappaB in the IL-8 promoter region resulted in a lower luciferase activity after HMGB1 stimulation.
CONCLUSION
The proinflammatory effects of HMGB1, particularly on IL-8, are mediated by both NF-kappaB and AP-1.
MeSH Terms
-
Acute Lung Injury
Animals
Binding Sites
CCAAT-Enhancer-Binding Protein-beta
Cytokines
Enzyme-Linked Immunosorbent Assay
HMGB1 Protein*
Inflammation
Injections, Intraperitoneal
Interleukin-8*
Luciferases
Lung
Mice
Negotiating
Neutrophils
NF-kappa B
Peritoneum
Promoter Regions, Genetic
Transcription Factor AP-1
Tumor Necrosis Factor-alpha
CCAAT-Enhancer-Binding Protein-beta
Cytokines
HMGB1 Protein
Interleukin-8
Luciferases
NF-kappa B
Transcription Factor AP-1
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973. 38:14–19.2. Ferrari S, Finelli P, Rocchi M, Bianchi ME. The active gene that encodes human high mobility group 1 protein (HMG1) contains introns and maps to chromosome 13. Genomics. 1996. 35:367–371.3. Hardman CH, Broadhurst RW, Raine ARC, Grasser KD, Thomas JO, Laue ED. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry. 1995. 34:16596–16607.4. Mosevitsky MI, Novitskaya VA, Iogannsen MG, Zabezhinsky MA. Tissue specificity of nucleo- cytoplasmic distribution of HMG1 and HMG2 proteins and their probable functions. Eur J Biochem. 1989. 185:303–310.5. Landsman D, Bustin M. A signature for the HMG-1 box DNA-binding proteins. Bioassays. 1993. 15:539–546.6. Calogero S, Grassi F, Aguzzi A, Voigtlander T, Ferrier P, Ferrari S, et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycemia in newborn mice. Nat Genet. 1999. 22:276–280.7. Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999. 285:248–251.8. Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003. 22:5551–5560.9. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002. 418:191–195.10. Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002. 54:1615–1625.11. Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptor 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004. 279:7370–7377.12. Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000. 192:565–570.13. Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003. 284:C870–C879.14. Angus DC, Linde-Zwirble WT, Lindicker J, Clemont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001. 29:1303–1310.15. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003. 348:1546–1554.16. Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med. 2003. 31:S276–S284.17. Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, et al. Contribution of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004. 170:1310–1316.18. Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung injury. J Immunol. 2000. 165:2950–2954.19. Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Phyisiol Lung Cell Mol Physiol. 2005. 288:L958–L965.20. Yang H, Ochani M, Li JH, Qiang X, Tanovic M, Harris HE, et al. Reversing established sepsis with antagonists of endogenous HMGB1. Proc Natl Acad Sci USA. 2004. 101:296–301.21. Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002. 99:12351–12356.22. Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L1137–L1145.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HMGB1-Binding Heptamer Confers Anti-Inflammatory Effects in Primary Microglia Culture
- Kinetics of HMGB1 level changes in a canine endotoxemia model
- Increased Serum High Mobility Group Box 1 (HMGB1) Concentration and the Altered Expression of HMGB1 and Its Receptor Advanced Glycation Endproducts in Pemphigus
- The Effects of Remifentanil on Expression of High Mobility Group Box 1 in Septic Rats
- Eosinophils Modulate CD4+ T Cell Responses via High Mobility Group Box-1 in the Pathogenesis of Asthma