J Korean Med Sci.
2014 Feb;29(2):230-237. 10.3346/jkms.2014.29.2.230.
Tempol Attenuates Renal Fibrosis in Mice with Unilateral Ureteral Obstruction: The Role of PI3K-Akt-FoxO3a Signaling
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. imkidney@catholic.ac.kr
- 2Division of Nephrology, Department of Internal Medicine, Incheon St. Mary's Hospital, Incheon, Korea.
- KMID: 1789980
- DOI: http://doi.org/10.3346/jkms.2014.29.2.230
Abstract
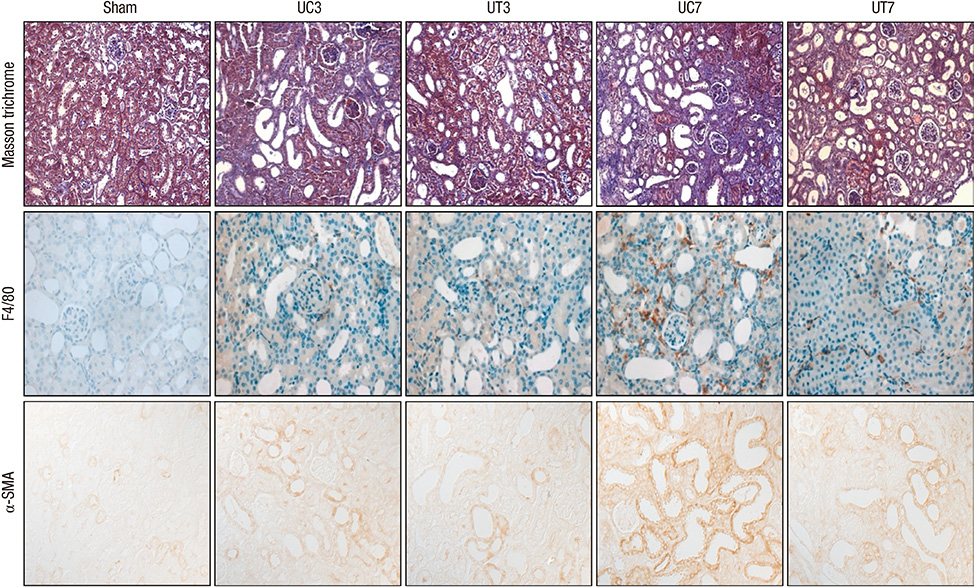
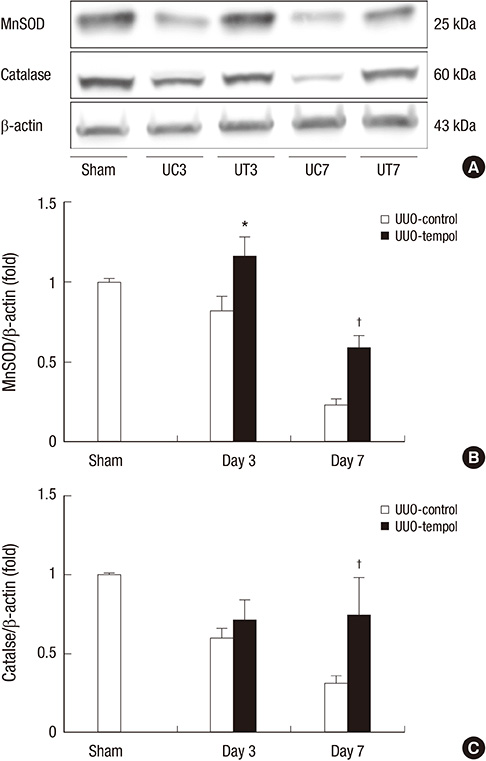
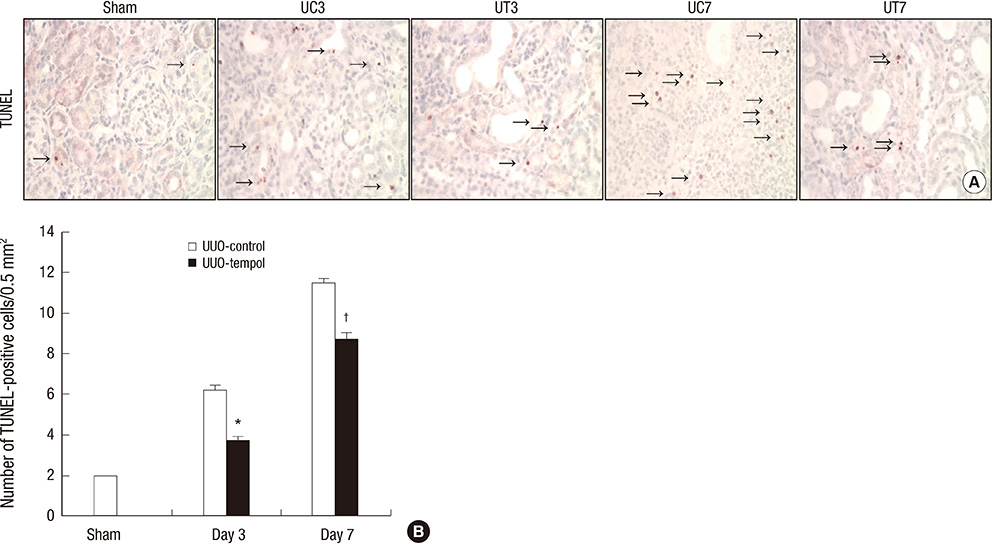
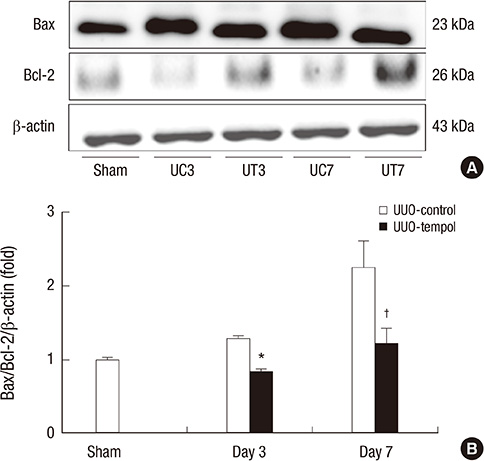
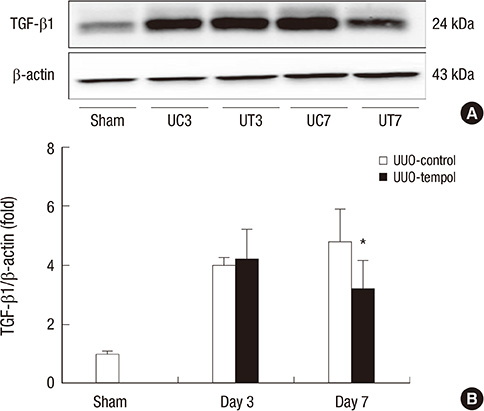
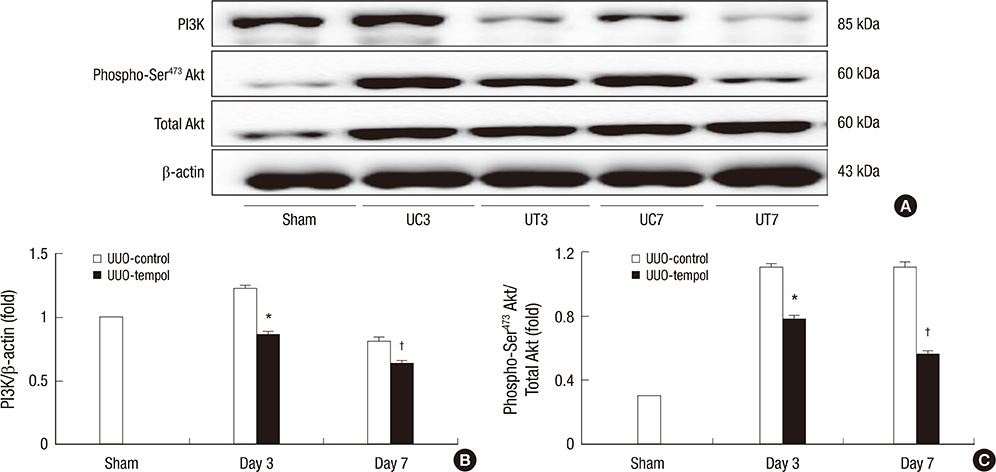
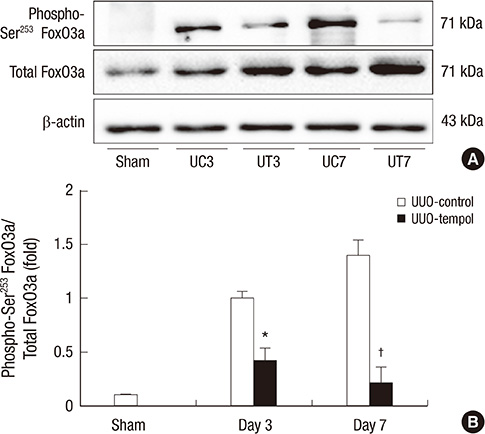
- This study investigated whether tempol, an anti-oxidant, protects against renal injury by modulating phosphatidylinositol 3-kinase (PI3K)-Akt-Forkhead homeobox O (FoxO) signaling. Mice received unilateral ureteral obstruction (UUO) surgery with or without administration of tempol. We evaluated renal damage, oxidative stress and the expression of PI3K, Akt, FoxO3a and their target molecules including manganese superoxide dismutase (MnSOD), catalase, Bax, and Bcl-2 on day 3 and day 7 after UUO. Tubulointerstitial fibrosis, collagen deposition, alpha-smooth muscle actin-positive area, and F4/80-positive macrophage infiltration were significantly lower in tempol-treated mice compared with control mice. The expression of PI3K, phosphorylated Akt, and phosphorylated FoxO3a markedly decreased in tempol-treated mice compared with control mice. Tempol prominently increased the expressions of MnSOD and catalase, and decreased the production of hydrogen peroxide and lipid peroxidation in the obstructed kidneys. Significantly less apoptosis, a lower ratio of Bax to Bcl-2 expression and fewer apoptotic cells in TUNEL staining, and decreased expression of transforming growth factor-beta1 were observed in the obstructed kidneys from tempol-treated mice compared with those from control mice. Tempol attenuates oxidative stress, inflammation, and fibrosis in the obstructed kidneys of UUO mice, and the modulation of PI3K-Akt-FoxO3a signaling may be involved in this pathogenesis.
Keyword
MeSH Terms
-
Animals
Antioxidants/pharmacology/therapeutic use
Collagen/metabolism
Cyclic N-Oxides/*pharmacology/therapeutic use
Fibrosis
Forkhead Transcription Factors/*metabolism
Hydrogen Peroxide/metabolism
Kidney Diseases/drug therapy/metabolism/pathology
Lipid Peroxidation
Male
Mice
Mice, Inbred C57BL
Oxidative Stress/drug effects
Phosphatidylinositol 3-Kinases/*metabolism
Phosphorylation/drug effects
Proto-Oncogene Proteins c-akt/*metabolism
Severity of Illness Index
Signal Transduction/*drug effects
Spin Labels
Superoxide Dismutase/metabolism
Ureteral Obstruction/complications/drug therapy/*metabolism/pathology
Antioxidants
Cyclic N-Oxides
Forkhead Transcription Factors
Spin Labels
Collagen
Hydrogen Peroxide
Superoxide Dismutase
Phosphatidylinositol 3-Kinases
Proto-Oncogene Proteins c-akt
Figure
Reference
-
1. Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004; 13:93–99.2. Himmelfarb J. Linking oxidative stress and inflammation in kidney disease: which is the chicken and which is the egg? Semin Dial. 2004; 17:449–454.3. Percy C, Pat B, Poronnik P, Gobe G. Role of oxidative stress in age-associated chronic kidney pathologies. Adv Chronic Kidney Dis. 2005; 12:78–83.4. Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011; 14:593–605.5. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011; 1813:1978–1986.6. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999; 96:857–868.7. Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002; 419:316–321.8. Van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007; 8:440–450.9. Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005; 12:353–365.10. Matsuo S, López-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int. 2005; 67:2221–2238.11. Kim J, Kil IS, Seok YM, Yang ES, Kim DK, Lim DG, Park JW, Bonventre JV, Park KM. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice: a role for manganese superoxide dismutase. J Biol Chem. 2006; 281:20349–20356.12. Chung HW, Lim JH, Kim MY, Shin SJ, Chung S, Choi BS, Kim HW, Kim YS, Park CW, Chang YS. High-fat diet-induced renal cell apoptosis and oxidative stress in spontaneously hypertensive rat are ameliorated by fenofibrate through the PPARα-FoxO3a-PGC-1α pathway. Nephrol Dial Transplant. 2012; 27:2213–2225.13. Luan J, Li W, Han J, Zhang W, Gong H, Ma R. Renal protection of in vivo administration of tempol in streptozotocin-induced diabetic rats. J Pharmacol Sci. 2012; 119:167–176.14. Peixoto EB, Papadimitriou A, Lopes de Faria JM, Lopes de Faria JB. Tempol reduces podocyte apoptosis via PARP signaling pathway in experimental diabetes mellitus. Nephron Exp Nephrol. 2012; 120:e81–e90.15. Rodriguez F, Lopez B, Perez C, Fenoy FJ, Hernandez I, Stec DE, Li Volti G, Salom MG. Chronic tempol treatment attenuates the renal hemodynamic effects induced by a heme oxygenase inhibitor in streptozotocin diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2011; 301:R1540–R1548.16. Chung S, Park CW, Shin SJ, Lim JH, Chung HW, Youn DY, Kim HW, Kim BS, Lee JH, Kim GH, et al. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol Dial Transplant. 2010; 25:389–399.17. Liu Y, Tang L, Chen B. Effects of antioxidant gene therapy on retinal neurons and oxidative stress in a model of retinal ischemia/reperfusion. Free Radic Biol Med. 2012; 52:909–915.18. Maiese K, Chong ZZ, Hou J, Shang YC. The "O" class: crafting clinical care with FoxO transcription factors. Adv Exp Med Biol. 2009; 665:242–260.19. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–619.20. Kojima T, Norose T, Tsuchiya K, Sakamoto K. Mouse 3T3-L1 cells acquire resistance against oxidative stress as the adipocytes differentiate via the transcription factor FoxO. Apoptosis. 2010; 15:83–93.21. Peng SL. Forkhead transcription factors in chronic inflammation. Int J Biochem Cell Biol. 2010; 42:482–485.22. Boor P, Celec P, Martin IV, Villa L, Hodosy J, Klenovicsová K, Esposito C, Schäfer S, Albrecht-Küpper B, Ostendorf T, et al. The peroxisome proliferator-activated receptor-α agonist, BAY PP1, attenuates renal fibrosis in rats. Kidney Int. 2011; 80:1182–1197.23. Jia YT, Wei W, Ma B, Xu Y, Liu WJ, Wang Y, Lv KY, Tang HT, Wei D, Xia ZF. Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J Immunol. 2007; 179:7808–7819.24. Ho KK, McGuire VA, Koo CY, Muir KW, de Olano N, Maifoshie E, Kelly DJ, McGovern UB, Monteiro LJ, Gomes AR, et al. Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin. J Biol Chem. 2012; 287:1545–1555.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Phosphorylation of PI3K/Akt/mTOR in the Obstructed Kidney of Rats with Unilateral Ureteral Obstruction
- Notch signaling in the collecting duct regulates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction in mice
- L-carnitine treatment attenuates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction
- Pathogenesis and management of renal fibrosis induced by unilateral ureteral obstruction
- Alantolactone Attenuates Renal Fibrosis via Inhibition of Transforming Growth Factor β/Smad3 Signaling Pathway