Chonnam Med J.
2013 Dec;49(3):108-112. 10.4068/cmj.2013.49.3.108.
Increased Phosphorylation of PI3K/Akt/mTOR in the Obstructed Kidney of Rats with Unilateral Ureteral Obstruction
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. skimw@chonnam.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2048814
- DOI: http://doi.org/10.4068/cmj.2013.49.3.108
Abstract
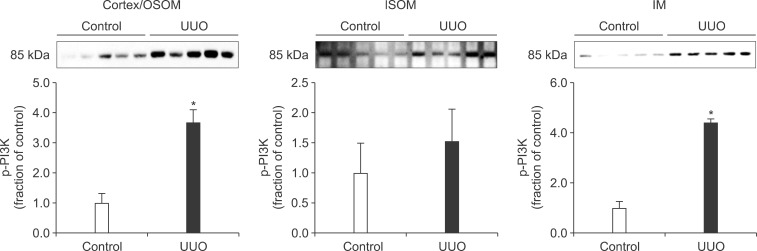
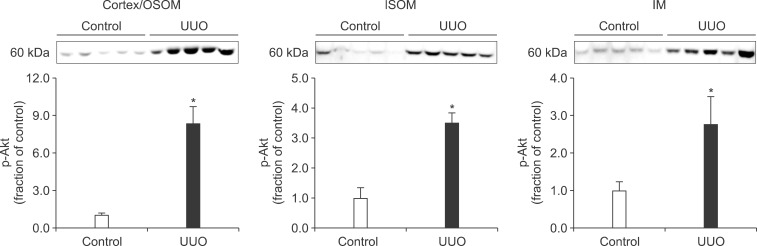
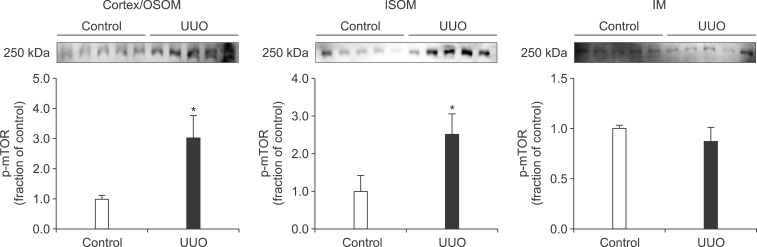
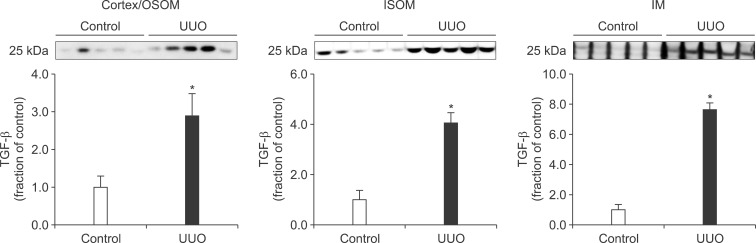
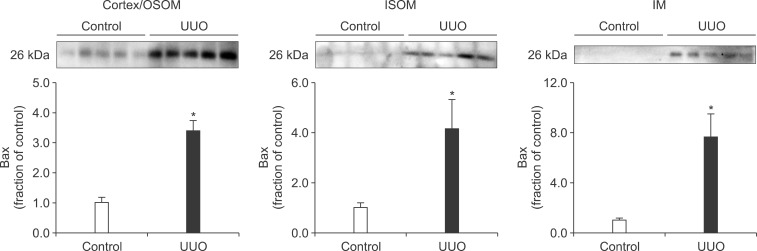
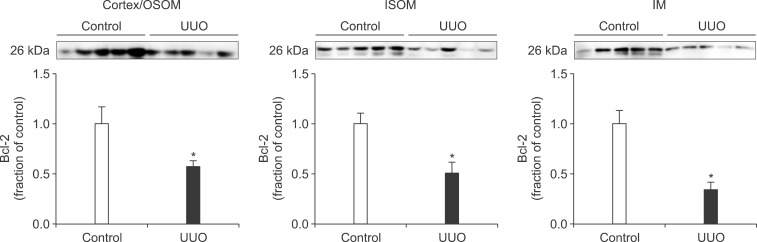
- The present study aimed to investigate changes in the mammalian target of rapamycin (mTOR) signaling pathway in the obstructed kidney of rats with unilateral ureteral obstruction (UUO). Male Sprague-Dawley rats were unilaterally obstructed by ligation of the left proximal ureter for 7 days. Control rats were treated in the same way except that no ligature was made. The expression levels of phosphorylated phosphatidylinositol 3-kinase (PI3K), Akt, and mTOR were determined in the kidney by semiquantitative immunoblotting. The protein expression levels of transforming growth factor (TGF)-beta1, Bax, and Bcl-2 were also determined in the kidney. The phosphorylation of PI3K, Akt, and mTOR was increased in the kidney of ureteral obstruction rats compared with the control. In the obstructed kidney, the protein expression of TGF-beta1 and Bax was also increased, whereas Bcl-2 expression was decreased. In conclusion, the phosphorylation of PI3K/Akt/mTOR was increased in the obstructed kidney of rats with UUO.
Keyword
MeSH Terms
-
Animals
Apoptosis
Fibrosis
Humans
Immunoblotting
Kidney*
Ligation
Male
Phosphatidylinositol 3-Kinase
Phosphorylation*
Rats*
Rats, Sprague-Dawley
Sirolimus
Transforming Growth Factor beta1
Transforming Growth Factors
Ureter*
Ureteral Obstruction*
Phosphatidylinositol 3-Kinase
Sirolimus
Transforming Growth Factor beta1
Transforming Growth Factors
Figure
Reference
-
1. Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002; 283:F861–F875. PMID: 12372761.2. Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycinreceptor complex. Nature. 1994; 369:756–758. PMID: 8008069.3. Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994; 78:35–43. PMID: 7518356.4. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011; 12:21–35. PMID: 21157483.5. Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009; 20:2493–2502. PMID: 19875810.6. Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. I. The signaling pathway. Am J Physiol Renal Physiol. 2012; 303:F1–F10. PMID: 22419691.7. Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. II. Pathophysiology and therapeutic implications. Am J Physiol Renal Physiol. 2012; 303:F180–F191. PMID: 22496407.8. Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, et al. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol. 2006; 17:1395–1404. PMID: 16597691.9. Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, et al. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol. 2007; 27:495–502. PMID: 17671379.10. Mori H, Inoki K, Masutani K, Wakabayashi Y, Komai K, Nakagawa R, et al. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun. 2009; 384:471–475. PMID: 19422788.11. Ma SK, Choi JS, Joo SY, Kim HY, Kim CS, Bae EH, et al. Activation of the Renal PI3K/Akt/mTOR Signaling Pathway in a DOCA-Salt Model of Hypertension. Chonnam Med J. 2012; 48:150–154. PMID: 23323219.12. Kaneto H, Morrissey J, Klahr S. Increased expression of TGF-beta 1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int. 1993; 44:313–321. PMID: 8377375.13. Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001; 17:615–675. PMID: 11687500.14. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004; 18:1926–1945. PMID: 15314020.15. Wittmann S, Daniel C, Stief A, Vogelbacher R, Amann K, Hugo C. Long-term treatment of sirolimus but not cyclosporine ameliorates diabetic nephropathy in the rat. Transplantation. 2009; 87:1290–1299. PMID: 19424027.16. Diekmann F, Rovira J, Carreras J, Arellano EM, Bañón-Maneus E, Ramírez-Bajo MJ, et al. Mammalian target of rapamycin inhibition halts the progression of proteinuria in a rat model of reduced renal mass. J Am Soc Nephrol. 2007; 18:2653–2660. PMID: 17804674.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Activation of the Renal PI3K/Akt/mTOR Signaling Pathway in a DOCA-Salt Model of Hypertension
- Tempol Attenuates Renal Fibrosis in Mice with Unilateral Ureteral Obstruction: The Role of PI3K-Akt-FoxO3a Signaling
- Significance of Renal Resistive Index in Children with Unilateral Ureteral Obstruction
- Role of Ammonia Transporters in the Kidney with Ureteral Obstruction
- New Application of the Partial Unilateral Ureteral Obstruction in Neonatal Rat Model