Chonnam Med J.
2012 Dec;48(3):150-154. 10.4068/cmj.2012.48.3.150.
Activation of the Renal PI3K/Akt/mTOR Signaling Pathway in a DOCA-Salt Model of Hypertension
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. skimw@chonnam.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2048803
- DOI: http://doi.org/10.4068/cmj.2012.48.3.150
Abstract
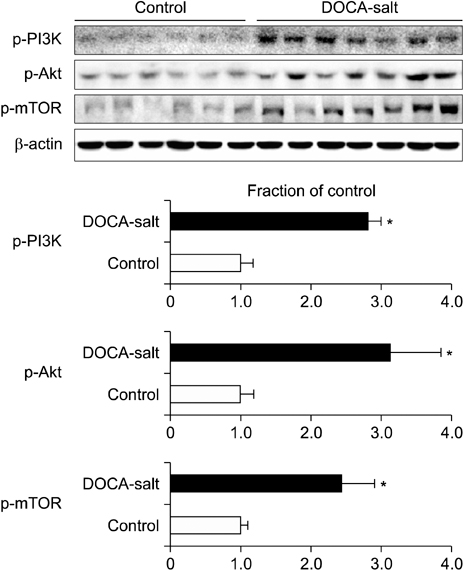
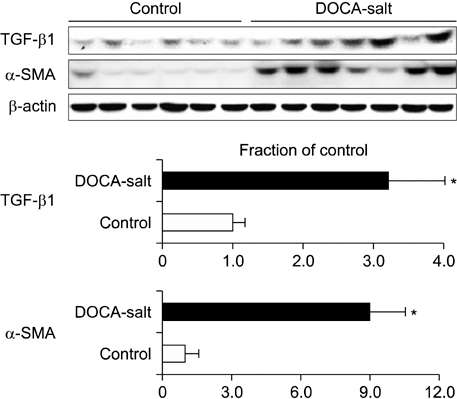
- The present study investigated the changes that occurred in the mammalian target of rapamycin (mTOR) signaling pathway in the kidney as a result of deoxycorticosterone acetate (DOCA)-salt hypertension. Rats were implanted with DOCA strips (200 mg/kg) 1 week after unilateral nephrectomy and were then supplied with 0.9% saline to drink. Four weeks after DOCA implantation, systolic blood pressure (SBP) was measured by use of the tail-cuff method. The expression levels of phosphorylated phosphatidylinositol-3-kinase (PI3K), Akt, and mTOR, as well as the protein expression levels of ED-1 and cyclooxygenase-2 (COX-2), transforming growth factor-beta1 (TGF-beta1), alpha-smooth muscle actin (SMA), caspase-3, Bax, and Bcl-2, were then examined in the kidney by semiquantitative immunoblotting. DOCA-salt hypertensive rats were found to have significantly increased SBP as well as an increased kidney weight-to-body weight ratio. Moreover, the phosphorylation of PI3K, Akt, and mTOR was increased in the kidney of DOCA-salt hypertensive rats compared with the control, as was the protein expression of ED-1, COX-2, TGF-beta1, and alpha-SMA. The expression levels of caspase-3 and Bax were increased significantly, whereas Bcl-2 expression was decreased. In conclusion, the phosphorylation of PI3K/Akt/mTOR was increased in the kidney of DOCA-salt hypertensive rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Gavras H, Brunner HR, Laragh JH, Vaughan ED Jr, Koss M, Cote LJ, et al. Malignant hypertension resulting from deoxycorticosterone acetate and salt excess: role of renin and sodium in vascular changes. Circ Res. 1975. 36:300–309.
Article2. Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction wtih the renin-angiotension system. Am J Hypertens. 2004. 17:597–603.
Article3. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol. 2002. 161:1773–1781.4. Lijnen PJ, Petrov VV, Fagard RH. Association between transforming growth factor-beta and hypertension. Am J Hypertens. 2003. 16:604–611.5. Liu Y. Rapamycin and chronic kidney disease: beyond the inhibition of inflammation. Kidney Int. 2006. 69:1925–1927.
Article6. Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009. 20:2493–2502.
Article7. Huang S, Zhang A, Ding G, Chen R. Aldosterone-induced mesangial cell proliferation is mediated by EGF receptor transactivation. Am J Physiol Renal Physiol. 2009. 296:F1323–F1333.
Article8. Bae EH, Kim IJ, Ma SK, Kim SW. Altered regulation of renal sodium transporters and natriuretic peptide system in DOCA-salt hypertensive rats. Regul Pept. 2009. 157:76–83.
Article9. Bae EH, Kim IJ, Park JW, Ma SK, Lee JU, Kim SW. Renoprotective effect of rosuvastatin in DOCA-salt hypertensive rats. Nephrol Dial Transplant. 2010. 25:1051–1059.
Article10. Bae EH, Kim IJ, Ma SK, Kim SW. Rosiglitazone prevents the progression of renal injury in DOCA-salt hypertensive rats. Hypertens Res. 2010. 33:255–262.
Article11. Iwazu Y, Muto S, Hirahara I, Fujisawa G, Takeda S, Kusano E. Matrix metalloproteinase 2 induces epithelial-mesenchymal transition in proximal tubules from the luminal side and progresses fibrosis in mineralocorticoid/salt-induced hypertensive rats. J Hypertens. 2011. 29:2440–2453.
Article12. Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, et al. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000. 58:2301–2313.
Article13. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, et al. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int. 2010. 77:1076–1085.
Article14. Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001. 17:615–675.
Article15. Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004. 18:1926–1945.
Article16. Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin Nephrol. 2003. 23:194–199.
Article17. Chen JK, Chen J, Neilson EG, Harris RC. Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol. 2005. 16:1384–1391.
Article18. Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes. 2007. 56:476–485.
Article19. Sataranatarajan K, Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, et al. Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol. 2007. 171:1733–1742.
Article20. Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, et al. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol. 2006. 17:1395–1404.
Article21. Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int. 2006. 69:2029–2036.
Article22. Diekmann F, Rovira J, Carreras J, Arellano EM, Bañón-Maneus E, Ramírez-Bajo MJ, et al. Mammalian target of rapamycin inhibition halts the progression of proteinuria in a rat model of reduced renal mass. J Am Soc Nephrol. 2007. 18:2653–2660.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isorhamnetin Alleviates Inflammation-Induced Crosstalk between Kynurenine Pathway and Gut Microbiota in Depressed Mice
- Inhibition of the interaction between Hippo/YAP and Akt signaling with ursolic acid and 3′3-diindolylmethane suppresses esophageal cancer tumorigenesis
- Time-Related Alterations of Endogenous Ouabain in DOCA-Salt Hypertensive Rats
- Melatonin Induces Akt Phosphorylation through Melatonin Receptor- and PI3K-Dependent Pathways in Primary Astrocytes
- Extracellular Vesicles from Adipose-Derived Stem Cells Promote Diabetic Wound Healing via the PI3K-AKT-mTOR-HIF-1a Signaling Pathway