Diabetes Metab J.
2024 Jan;48(1):72-82. 10.4093/dmj.2022.0231.
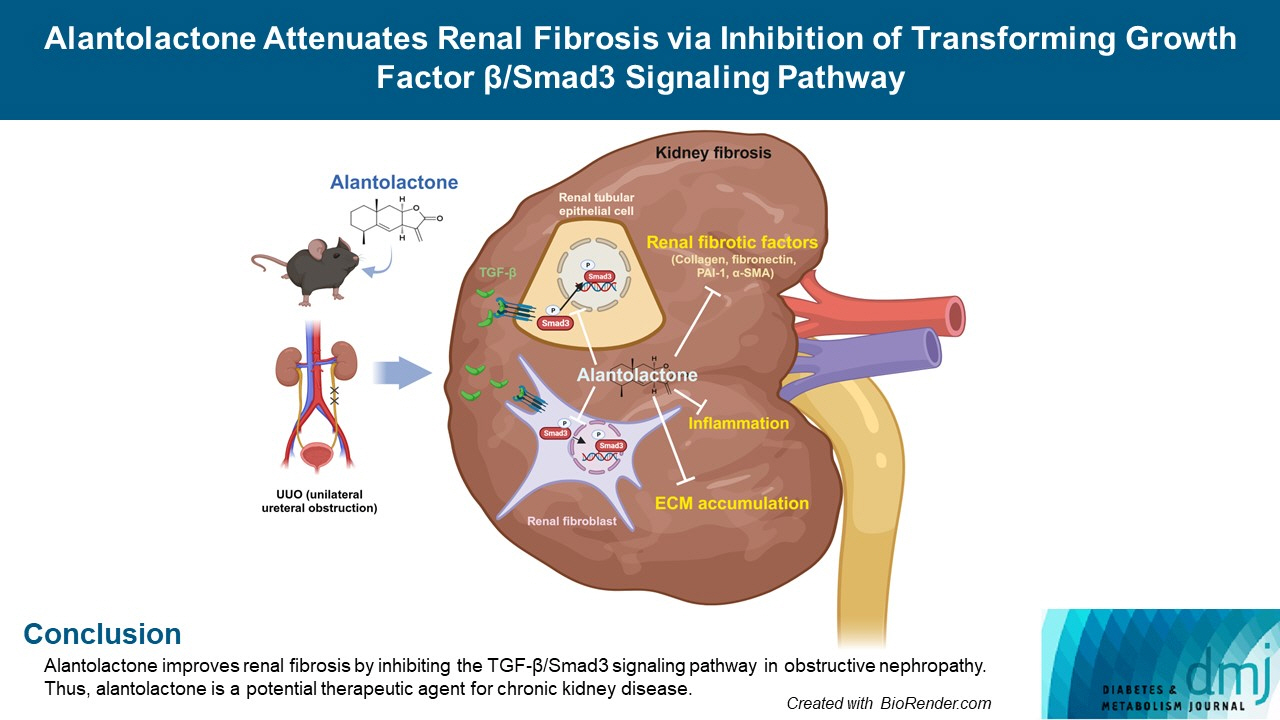
Alantolactone Attenuates Renal Fibrosis via Inhibition of Transforming Growth Factor β/Smad3 Signaling Pathway
- Affiliations
-
- 1Division of Biotechnology, Daegu Gyeongbuk Institute of Science and Technology, Daegu, Korea
- 2New Drug Development Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea
- KMID: 2551264
- DOI: http://doi.org/10.4093/dmj.2022.0231
Abstract
- Background
Renal fibrosis is characterized by the accumulation of extracellular matrix proteins and interstitial fibrosis. Alantolactone is known to exert anticancer, anti-inflammatory, antimicrobial and antifungal effects; however, its effects on renal fibrosis remains unknown. Here, we investigated whether alantolactone attenuates renal fibrosis in mice unilateral ureteral obstruction (UUO) and evaluated the effect of alantolactone on transforming growth factor (TGF) signaling pathway in renal cells
Methods
To evaluate the therapeutic effect of alantolactone, cell counting kit-8 (CCK-8) assay, histological staining, Western blot analysis, and real-time quantitative polymerase chain reaction were performed in UUO kidneys in vivo and in TGF-β-treated renal cells in vitro.
Results
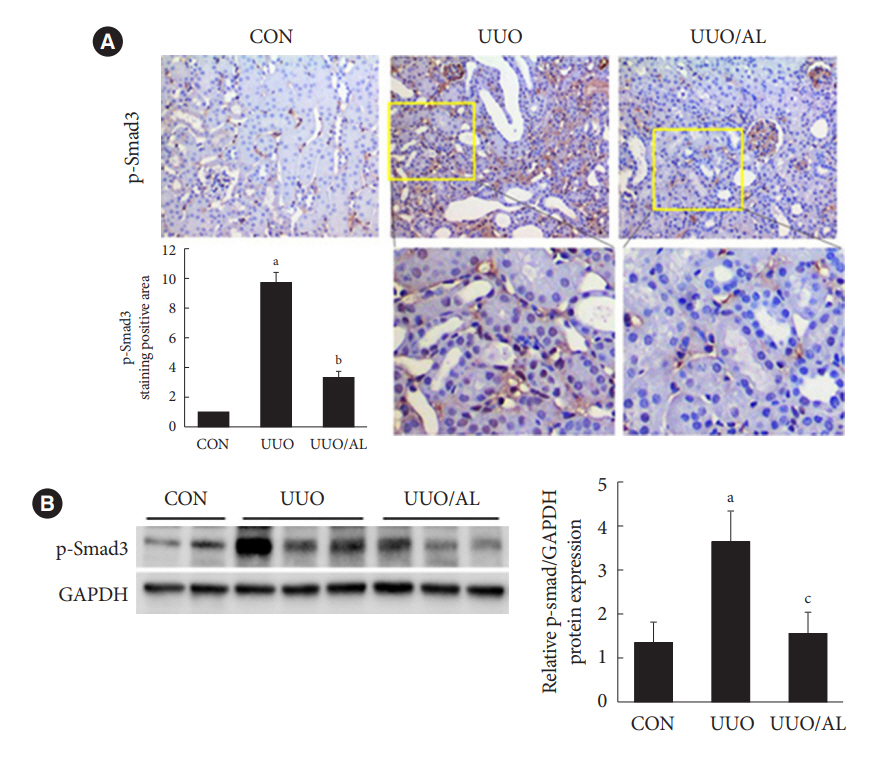
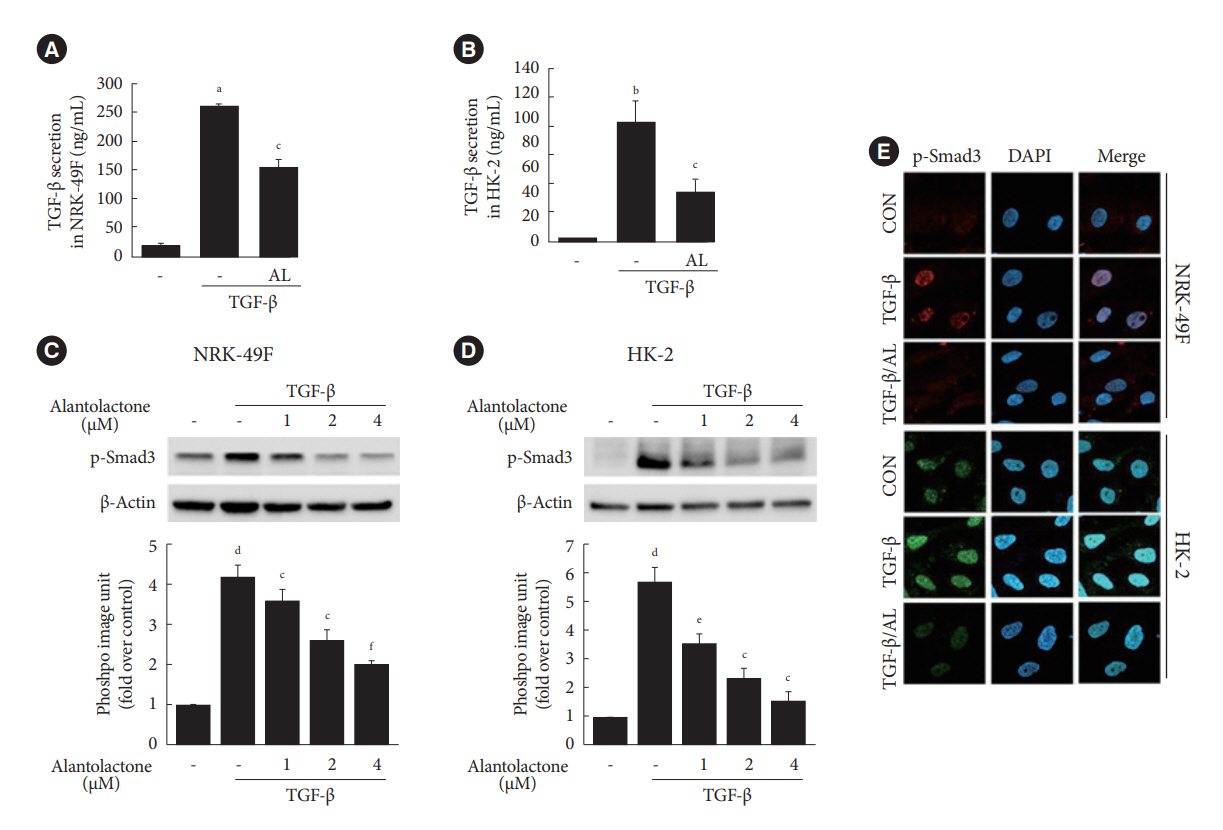
Alantolactone (0.25 to 4 µM) did not affect the viability of renal cells. Mice orally administered 5 mg/kg of alantolactone daily for 15 days did not show mortality or liver toxicity. Alantolactone decreased UUO-induced blood urea nitrogen and serum creatinine levels. In addition, it significantly alleviated renal tubulointerstitial damage and fibrosis and decreased collagen type I, fibronectin, and α-smooth muscle actin (α-SMA) expression in UUO kidneys. In NRK-49F cells, alantolactone inhibited TGF-βstimulated expression of fibronectin, collagen type I, plasminogen activator inhibitor-1 (PAI-1), and α-SMA. In HK-2 cells, alantolactone inhibited TGF-β-stimulated expression of collagen type I and PAI-1. Alantolactone inhibited UUO-induced phosphorylation of Smad3 in UUO kidneys. In addition, it not only decreased TGF-β secretion but also Smad3 phosphorylation and translocation to nucleus in both kidney cell lines.
Conclusion
Alantolactone improves renal fibrosis by inhibiting the TGF-β/Smad3 signaling pathway in obstructive nephropathy. Thus, alantolactone is a potential therapeutic agent for chronic kidney disease.
Figure
Reference
-
1. Grgic I, Duffield JS, Humphreys BD. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr Nephrol. 2012; 27:183–93.2. Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011; 7:684–96.3. Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010; 21:1819–34.4. Border WA, Okuda S, Languino LR, Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990; 37:689–95.5. Meng XM, Tang PM, Li J, Lan HY. TGF-β/Smad signaling in renal fibrosis. Front Physiol. 2015; 6:82.6. Nakamura T, Miller D, Ruoslahti E, Border WA. Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-beta 1. Kidney Int. 1992; 41:1213–21.7. Jung GS, Hwang YJ, Choi JH, Lee KM. Lin28a attenuates TGF-β-induced renal fibrosis. BMB Rep. 2020; 53:594–9.8. Roberts AB, Heine UI, Flanders KC, Sporn MB. Transforming growth factor-beta: major role in regulation of extracellular matrix. Ann N Y Acad Sci. 1990; 580:225–32.9. Miyazono K. TGF-beta signaling by Smad proteins. Cytokine Growth Factor Rev. 2000; 11:15–22.10. Hills CE, Squires PE. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011; 22:131–9.11. Li L, Emmett N, Mann D, Zhao X. Fenofibrate attenuates tubulointerstitial fibrosis and inflammation through suppression of nuclear factor-κB and transforming growth factor-β1/Smad3 in diabetic nephropathy. Exp Biol Med (Maywood). 2010; 235:383–91.12. Ai J, Nie J, He J, Guo Q, Li M, Lei Y, et al. GQ5 hinders renal fibrosis in obstructive nephropathy by selectively inhibiting TGF-β-induced Smad3 phosphorylation. J Am Soc Nephrol. 2015; 26:1827–38.13. Li J, Campanale NV, Liang RJ, Deane JA, Bertram JF, Ricardo SD. Inhibition of p38 mitogen-activated protein kinase and transforming growth factor-beta1/Smad signaling pathways modulates the development of fibrosis in adriamycin-induced nephropathy. Am J Pathol. 2006; 169:1527–40.14. Nam HK, Lee SJ, Kim MH, Rho JH, Son YK, Lee SM, et al. Rosuvastatin attenuates inflammation, apoptosis and fibrosis in a rat model of cyclosporine-induced nephropathy. Am J Nephrol. 2013; 37:7–15.15. Hoffmann D. Medical herbalism: the science and practice of herbal medicine. Fairfield: Healing Arts Press;2003. p. 672.16. Rasul A, Khan M, Ali M, Li J, Li X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. ScientificWorldJournal. 2013; 2013:248532.17. Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, et al. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol. 2012; 14:375–83.18. Seo JY, Lim SS, Kim JR, Lim JS, Ha YR, Lee IA, et al. Nrf2-mediated induction of detoxifying enzymes by alantolactone present in Inula helenium. Phytother Res. 2008; 22:1500–5.19. Zhu Y, Ling Y, Wang X. Alantolactone mitigates renal injury induced by diabetes via inhibition of high glucose-mediated inflammatory response and macrophage infiltration. Immunopharmacol Immunotoxicol. 2020; 42:84–92.20. Seo JY, Lim SS, Kim J, Lee KW, Kim JS. Alantolactone and isoalantolactone prevent amyloid β25-35-induced toxicity in mouse cortical neurons and scopolamine-induced cognitive impairment in mice. Phytother Res. 2017; 31:801–11.21. Zhao WY, Luan ZL, Liu TT, Ming WH, Huo XK, Huang HL, et al. Inula japonica ameliorated bleomycin-induced pulmonary fibrosis via inhibiting soluble epoxide hydrolase. Bioorg Chem. 2020; 102:104065.22. Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol. 2011; 22:890–901.23. Xu R, Peng Y, Wang M, Li X. Intestinal absorption of isoalantolactone and alantolactone, two sesquiterpene lactones from Radix Inulae, using Caco-2 cells. Eur J Drug Metab Pharmacokinet. 2019; 44:295–303.24. Xu R, Zhou G, Peng Y, Wang M, Li X. Pharmacokinetics, tissue distribution and excretion of isoalantolactone and alantolactone in rats after oral administration of Radix Inulae extract. Molecules. 2015; 20:7719–36.25. Lee JY, Kim SB, Chun J, Song KH, Kim YS, Chung SJ, et al. High body clearance and low oral bioavailability of alantolactone, isolated from Inula helenium, in rats: extensive hepatic metabolism and low stability in gastrointestinal fluids. Biopharm Drug Dispos. 2016; 37:156–67.26. Jiang Y, Xu H, Wang J. Alantolactone induces apoptosis of human cervical cancer cells via reactive oxygen species generation, glutathione depletion and inhibition of the Bcl-2/Bax signaling pathway. Oncol Lett. 2016; 11:4203–7.27. Wang X, Yu Z, Wang C, Cheng W, Tian X, Huo X, et al. Alantolactone, a natural sesquiterpene lactone, has potent antitumor activity against glioblastoma by targeting IKKβ kinase activity and interrupting NF-κB/COX-2-mediated signaling cascades. J Exp Clin Cancer Res. 2017; 36:93.28. Liu YR, Cai QY, Gao YG, Luan X, Guan YY, Lu Q, et al. Alantolactone, a sesquiterpene lactone, inhibits breast cancer growth by antiangiogenic activity via blocking VEGFR2 signaling. Phytother Res. 2018; 32:643–50.29. Yin C, Dai X, Huang X, Zhu W, Chen X, Zhou Q, et al. Alantolactone promotes ER stress-mediated apoptosis by inhibition of TrxR1 in triple-negative breast cancer cell lines and in a mouse model. J Cell Mol Med. 2019; 23:2194–206.30. Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009; 75:1145–52.31. Tashiro K, Tamada S, Kuwabara N, Komiya T, Takekida K, Asai T, et al. Attenuation of renal fibrosis by proteasome inhibition in rat obstructive nephropathy: possible role of nuclear factor kappaB. Int J Mol Med. 2003; 12:587–92.32. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003; 112:1486–94.33. Meng XM, Huang XR, Xiao J, Chen HY, Zhong X, Chung AC, et al. Diverse roles of TGF-β receptor II in renal fibrosis and inflammation in vivo and in vitro. J Pathol. 2012; 227:175–88.34. Samarakoon R, Overstreet JM, Higgins SP, Higgins PJ. TGF-β1 → SMAD/p53/USF2 → PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res. 2012; 347:117–28.35. Khan M, Li T, Ahmad Khan MK, Rasul A, Nawaz F, Sun M, et al. Alantolactone induces apoptosis in HepG2 cells through GSH depletion, inhibition of STAT3 activation, and mitochondrial dysfunction. Biomed Res Int. 2013; 2013:719858.36. Park JY, Yoo KD, Bae E, Kim KH, Lee JW, Shin SJ, et al. Blockade of STAT3 signaling alleviates the progression of acute kidney injury to chronic kidney disease through antiapoptosis. Am J Physiol Renal Physiol. 2022; 322:F553–72.37. Qin CZ, Lv QL, Wu NY, Cheng L, Chu YC, Chu TY, et al. Mechanism-based inhibition of alantolactone on human cytochrome P450 3A4 in vitro and activity of hepatic cytochrome P450 in mice. J Ethnopharmacol. 2015; 168:146–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Smads as therapeutic targets for chronic kidney disease
- TGF-beta-activated kinase-1: New insights into the mechanism of TGF-beta signaling and kidney disease
- Hypoxia-Inducible Factor 1α Regulates the Transforming Growth Factor β1/SMAD Family Member 3 Pathway to Promote Breast Cancer Progression
- Renal fibrosis
- Evogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice