Diabetes Metab J.
2020 Feb;44(1):186-192. 10.4093/dmj.2018.0271.
Evogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea. kpark@knu.ac.kr, exc4932@hanmail.net
- 2Department of Biomedical Science, Graduate School, Kyungpook National University, Daegu, Korea.
- 3BK21 Plus KNU Biomedical Convergence Program, Kyungpook National University, Daegu, Korea.
- 4New Drug Development Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea.
- KMID: 2470966
- DOI: http://doi.org/10.4093/dmj.2018.0271
Abstract
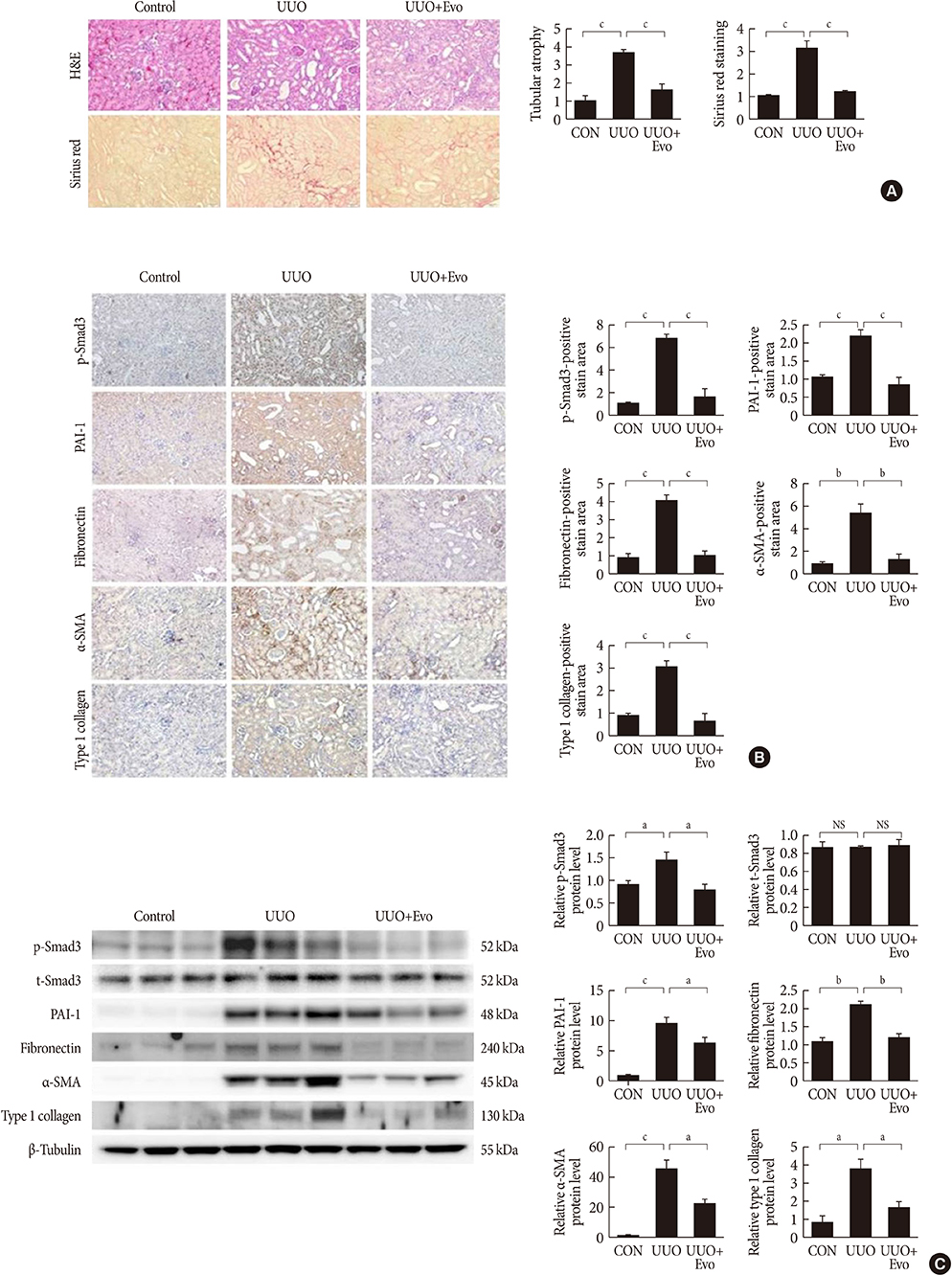
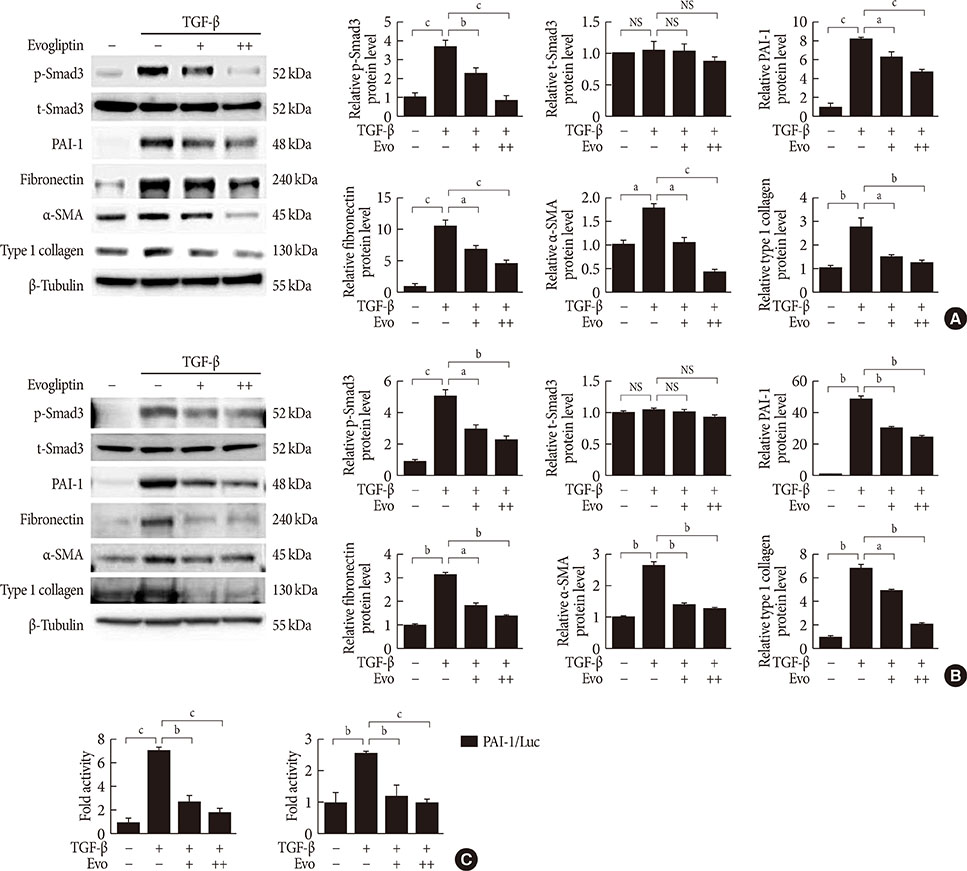
- Renal fibrosis is considered to be the final common outcome of chronic kidney disease. Dipeptidyl peptidase-4 (DPP-4) inhibitors have demonstrated protective effects against diabetic kidney disease. However, the anti-fibrotic effect of evogliptin, a DPP-4 inhibitor, has not been studied. Here, we report the beneficial effects of evogliptin on unilateral ureteral obstruction (UUO)-induced renal fibrosis in mice. Evogliptin attenuated UUO-induced renal atrophy and tubulointerstitial fibrosis. Immunohistochemistry and Western blotting demonstrated that evogliptin treatment inhibits pro-fibrotic gene expressions and extracellular matrix production. In vitro findings showed that the beneficial effects of evogliptin on renal fibrosis are mediated by inhibition of the transforming growth factor-β/Smad3 signaling pathway. The present study demonstrates that evogliptin is protective against UUO-induced renal fibrosis, suggesting that its clinical applications could extend to the treatment of kidney disease of non-diabetic origin.
Keyword
MeSH Terms
-
Animals
Atrophy
Blotting, Western
Diabetic Nephropathies
Dipeptidyl-Peptidase IV Inhibitors
Extracellular Matrix
Fibrosis*
Gene Expression
Immunohistochemistry
In Vitro Techniques
Kidney Diseases
Kidney Failure, Chronic
Mice*
Renal Insufficiency, Chronic
Transforming Growth Factor beta
Ureter*
Ureteral Obstruction*
Dipeptidyl-Peptidase IV Inhibitors
Transforming Growth Factor beta
Figure
Reference
-
1. Atkins RC, Zimmet P. IFKF World Kidney Day 2010 Steering Committee. Diabetes: diabetic kidney disease: act now or pay later. Nat Rev Nephrol. 2010; 6:134–136.2. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016; 12:325–338.
Article3. Lan HY, Chung AC. TGF-β/Smad signaling in kidney disease. Semin Nephrol. 2012; 32:236–243.
Article4. Salvo F, Moore N, Arnaud M, Robinson P, Raschi E, De Ponti F, Begaud B, Pariente A. Addition of dipeptidyl peptidase-4 inhibitors to sulphonylureas and risk of hypoglycaemia: systematic review and meta-analysis. BMJ. 2016; 353:i2231.
Article5. Hirakawa H, Zempo H, Ogawa M, Watanabe R, Suzuki J, Akazawa H, Komuro I, Isobe M. A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice. PLoS One. 2015; 10:e0119360.
Article6. Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, Douhara A, Moriya K, Kawaratani H, Shirai Y, Yoshii J, Yanase K, Kitade M, Namisaki T, Fukui H. Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J Gastroenterol. 2014; 49:481–491.
Article7. Suzuki T, Tada Y, Gladson S, Nishimura R, Shimomura I, Karasawa S, Tatsumi K, West J. Vildagliptin ameliorates pulmonary fibrosis in lipopolysaccharide-induced lung injury by inhibiting endothelial-to-mesenchymal transition. Respir Res. 2017; 18:177.
Article8. Eun Lee J, Kim JE, Lee MH, Song HK, Ghee JY, Kang YS, Min HS, Kim HW, Cha JJ, Han JY, Han SY, Cha DR. DA-1229, a dipeptidyl peptidase IV inhibitor, protects against renal injury by preventing podocyte damage in an animal model of progressive renal injury. Lab Invest. 2016; 96:547–560.
Article9. Tan X, Hu J. Evogliptin: a new dipeptidyl peptidase inhibitor for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2016; 17:1285–1293.
Article10. Min HS, Kim JE, Lee MH, Song HK, Kang YS, Lee MJ, Lee JE, Kim HW, Cha JJ, Chung YY, Hyun YY, Han JY, Cha DR. Dipeptidyl peptidase IV inhibitor protects against renal interstitial fibrosis in a mouse model of ureteral obstruction. Lab Invest. 2014; 94:598–607.
Article11. Kim MK, Chae YN, Kim HD, Yang EK, Cho EJ, Choi SH, Cheong YH, Kim HS, Kim HJ, Jo YW, Son MH, Kim SH, Shin CY. DA-1229, a novel and potent DPP4 inhibitor, improves insulin resistance and delays the onset of diabetes. Life Sci. 2012; 90:21–29.
Article12. Jung GS, Kim MK, Jung YA, Kim HS, Park IS, Min BH, Lee KU, Kim JG, Park KG, Lee IK. Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol. 2012; 23:73–85.
Article13. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002; 110:341–350.
Article14. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003; 112:1486–1494.15. Wang D, Zhang G, Chen X, Wei T, Liu C, Chen C, Gong Y, Wei Q. Sitagliptin ameliorates diabetic nephropathy by blocking TGF-β1/Smad signaling pathway. Int J Mol Med. 2018; 41:2784–2792.
Article16. Uchida T, Oda T, Matsubara H, Watanabe A, Takechi H, Oshima N, Sakurai Y, Kumagai H. Renoprotective effects of a dipeptidyl peptidase 4 inhibitor in a mouse model of progressive renal fibrosis. Ren Fail. 2017; 39:340–349.
Article17. Jung GS, Jeon JH, Choe MS, Kim SW, Lee IK, Kim MK, Park KG. Renoprotective effect of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in streptozotocin-induced type 1 diabetic mice. Diabetes Metab J. 2016; 40:211–221.
Article18. Shi S, Srivastava SP, Kanasaki M, He J, Kitada M, Nagai T, Nitta K, Takagi S, Kanasaki K, Koya D. Interactions of DPP-4 and integrin β1 influences endothelial-to-mesenchymal transition. Kidney Int. 2015; 88:479–489.
Article19. Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK, Shao YM, Park TS. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2012; 340:248–255.
Article20. Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014; 63:2120–2131.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of evogliptin and cenicriviroc against nonalcoholic steatohepatitis in mice: a comparative study
- Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Thalidomide and a Dipeptidyl Peptidase 4 Inhibitor in a Rat Model of Experimental Autoimmune Myocarditis
- Lobeglitazone, a Novel Peroxisome Proliferator-Activated Receptor γ Agonist, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice