Diabetes Metab J.
2019 Dec;43(6):830-839. 10.4093/dmj.2018.0181.
Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea. kpark@knu.ac.kr
- 2New Drug Development Center, Daegu-Gyeongbuk Medical Innovation Foundation, Daegu, Korea. gsjung@dgmif.re.kr
- 3Department of Biomedical Science, Graduate School, Kyungpook National University, Daegu, Korea.
- 4BK21 Plus KNU Biomedical Convergence Program, Kyungpook National University, Daegu, Korea.
- KMID: 2466504
- DOI: http://doi.org/10.4093/dmj.2018.0181
Abstract
- BACKGROUND
The hypoglycemic drugs dipeptidyl peptidase-4 (DPP-4) inhibitors have proven protective effects on diabetic kidney disease, including renal fibrosis. Although NOD-like receptor protein 3 (NLRP3) inflammasome activation is known to play an important role in the progression of renal fibrosis, the impact of DPP-4 inhibition on NLRP3-mediated inflammation while ameliorating renal fibrosis has not been fully elucidated. Here, we report that the renoprotective effect of gemigliptin is associated with a reduction in NLRP3-mediated inflammation in a murine model of renal fibrosis.
METHODS
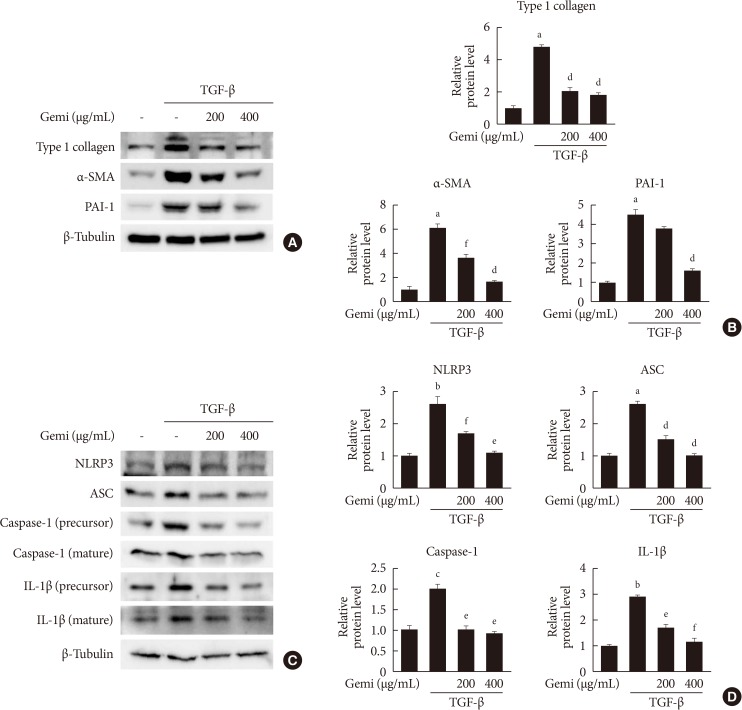
We examined the effects of gemigliptin on renal tubulointerstitial fibrosis induced in mice by unilateral ureteral obstruction (UUO). Using immunohistochemical and Western blot analysis, we quantitated components of the NLRP3 inflammasome in kidneys with and without gemigliptin treatment, and in vitro in human kidney tubular epithelial human renal proximal tubule cells (HK-2) cells, we further analyzed the effect of gemigliptin on transforming growth factor-β (TGF-β)-stimulated production of profibrotic proteins.
RESULTS
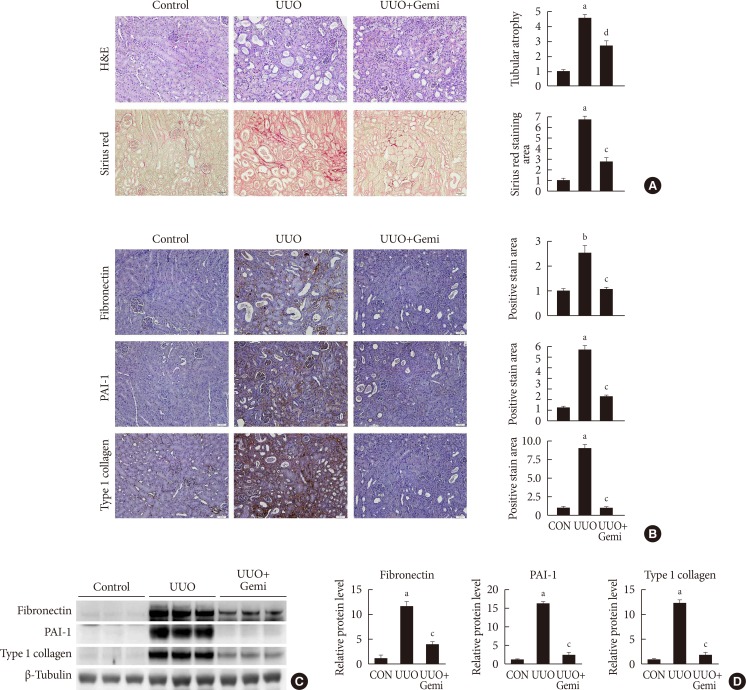
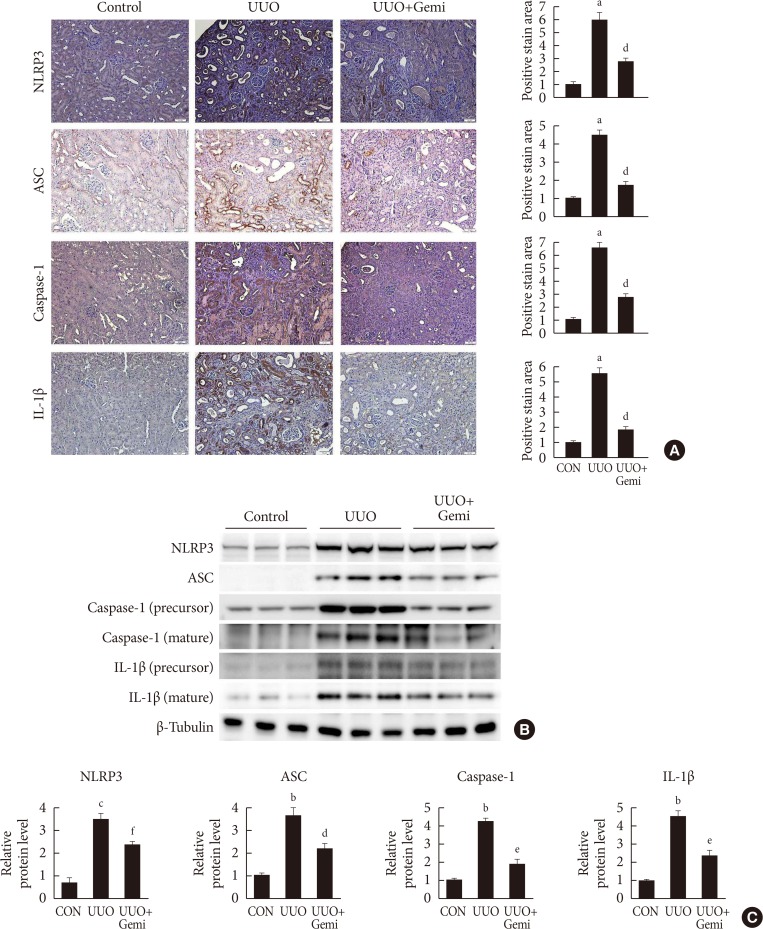
Immunohistological examination revealed that gemigliptin ameliorated UUO-induced tubular atrophy and renal fibrosis. Gemigliptin-treated kidneys showed a reduction in levels of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase-1, and interleukin-1β, which had all been markedly increased by UUO. In line with the in vivo
results
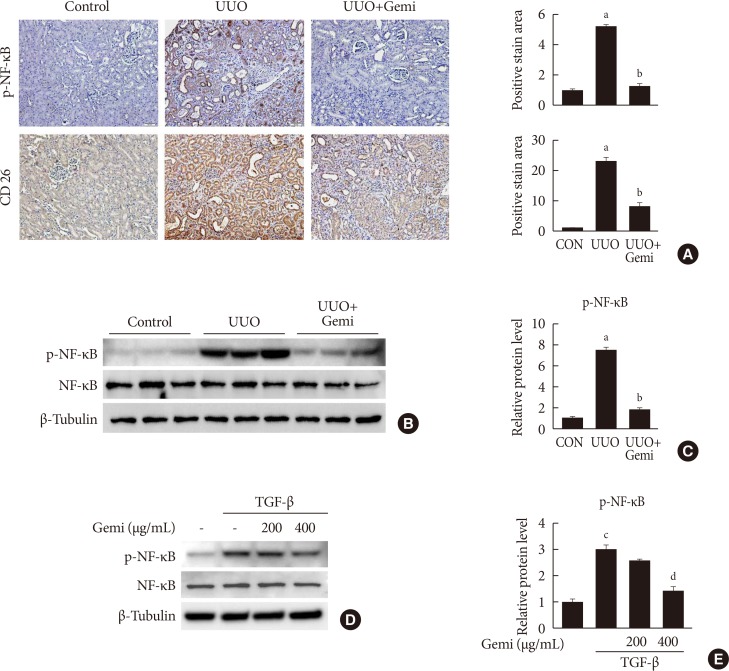
, TGF-β markedly increased NLRP3 inflammasome markers, which were attenuated by gemigliptin treatment. Furthermore, gemigliptin treatment attenuated phosphorylated nuclear factor-κB levels, which had been increased in the UUO kidney as well as in TGF-β-treated cultured renal cells.
CONCLUSION
The present study shows that activation of the NLRP3 inflammasome contributes to UUO-induced renal fibrosis and the renoprotective effect of gemigliptin is associated with attenuation of NLRP3 inflammasome activation.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim KS, Park SW, Cho YW, Kim SK. Higher prevalence and progression rate of chronic kidney disease in elderly patients with type 2 diabetes mellitus. Diabetes Metab J. 2018; 42:224–232. PMID: 29885112.
Article2. Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 2004; 43:9–17. PMID: 14964574.
Article3. Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010; 21:1732–1744. PMID: 20688930.
Article4. Gong W, Mao S, Yu J, Song J, Jia Z, Huang S, Zhang A. NLRP3 deletion protects against renal fibrosis and attenuates mitochondrial abnormality in mouse with 5/6 nephrectomy. Am J Physiol Renal Physiol. 2016; 310:F1081–F1088. PMID: 26887832.
Article5. Tsuprykov O, Ando R, Reichetzeder C, von Websky K, Antonenko V, Sharkovska Y, Chaykovska L, Rahnenführer J, Hasan AA, Tammen H, Alter M, Klein T, Ueda S, Yamagishi SI, Okuda S, Hocher B. The dipeptidyl peptidase inhibitor linagliptin and the angiotensin II receptor blocker telmisartan show renal benefit by different pathways in rats with 5/6 nephrectomy. Kidney Int. 2016; 89:1049–1061. PMID: 27083282.
Article6. Shi S, Koya D, Kanasaki K. Dipeptidyl peptidase-4 and kidney fibrosis in diabetes. Fibrogenesis Tissue Repair. 2016; 9:1. PMID: 26877767.
Article7. Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014; 63:2120–2131. PMID: 24574044.
Article8. Uchida T, Oda T, Matsubara H, Watanabe A, Takechi H, Oshima N, Sakurai Y, Kumagai H. Renoprotective effects of a dipeptidyl peptidase 4 inhibitor in a mouse model of progressive renal fibrosis. Ren Fail. 2017; 39:340–349. PMID: 28118775.
Article9. Jung GS, Jeon JH, Choe MS, Kim SW, Lee IK, Kim MK, Park KG. Renoprotective effect of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in streptozotocin-induced type 1 diabetic mice. Diabetes Metab J. 2016; 40:211–221. PMID: 27098503.
Article10. He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016; 41:1012–1021. PMID: 27669650.
Article11. Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011; 22:1007–1018. PMID: 21566058.
Article12. Chang A, Ko K, Clark MR. The emerging role of the inflammasome in kidney diseases. Curr Opin Nephrol Hypertens. 2014; 23:204–210. PMID: 24685591.
Article13. Wang W, Wang X, Chun J, Vilaysane A, Clark S, French G, Bracey NA, Trpkov K, Bonni S, Duff HJ, Beck PL, Muruve DA. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J Immunol. 2013; 190:1239–1249. PMID: 23264657.
Article14. Lorenz G, Darisipudi MN, Anders HJ. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant. 2014; 29:41–48. PMID: 24026244.
Article15. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002; 10:417–426. PMID: 12191486.16. Choi SH, Leem J, Park S, Lee CK, Park KG, Lee IK. Gemigliptin ameliorates Western-diet-induced metabolic syndrome in mice. Can J Physiol Pharmacol. 2017; 95:129–139. PMID: 27918207.
Article17. Choi SH, Park S, Oh CJ, Leem J, Park KG, Lee IK. Dipeptidyl peptidase-4 inhibition by gemigliptin prevents abnormal vascular remodeling via NF-E2-related factor 2 activation. Vascul Pharmacol. 2015; 73:11–19. PMID: 26187356.
Article18. Jung GS, Kim MK, Jung YA, Kim HS, Park IS, Min BH, Lee KU, Kim JG, Park KG, Lee IK. Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol. 2012; 23:73–85. PMID: 22052058.
Article19. Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010; 21:1819–1834. PMID: 20864689.
Article20. Anders HJ, Lech M. NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int. 2013; 84:225–228. PMID: 23903414.
Article21. Ludwig-Portugall I, Bartok E, Dhana E, Evers BD, Primiano MJ, Hall JP, Franklin BS, Knolle PA, Hornung V, Hartmann G, Boor P, Latz E, Kurts C. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 2016; 90:525–539. PMID: 27262364.22. Birnbaum Y, Bajaj M, Qian J, Ye Y. Dipeptidyl peptidase-4 inhibition by saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diabetes Res Care. 2016; 4:e000227.
Article23. Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001; 107:1529–1536. PMID: 11413160.24. Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009; 1:a000034. PMID: 20066092.25. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017; 2.
Article26. Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005; 6:1087–1095. PMID: 16186825.
Article27. Kim JY, Omori E, Matsumoto K, Nunez G, Ninomiya-Tsuji J. TAK1 is a central mediator of NOD2 signaling in epidermal cells. J Biol Chem. 2008; 283:137–144. PMID: 17965022.
Article28. Avogaro A, Fadini GP. The effects of dipeptidyl peptidase-4 inhibition on microvascular diabetes complications. Diabetes Care. 2014; 37:2884–2894. PMID: 25249673.
Article29. Min HS, Kim JE, Lee MH, Song HK, Kang YS, Lee MJ, Lee JE, Kim HW, Cha JJ, Chung YY, Hyun YY, Han JY, Cha DR. Dipeptidyl peptidase IV inhibitor protects against renal interstitial fibrosis in a mouse model of ureteral obstruction. Lab Invest. 2014; 94:598–607. PMID: 24687121.
Article30. Zhong J, Maiseyeu A, Davis SN, Rajagopalan S. DPP4 in cardiometabolic disease: recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ Res. 2015; 116:1491–1504. PMID: 25858071.31. Fujita H, Taniai H, Murayama H, Ohshiro H, Hayashi H, Sato S, Kikuchi N, Komatsu T, Komatsu K, Komatsu K, Narita T, Yamada Y. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1α in type 2 diabetic patients with incipient nephropathy. Endocr J. 2014; 61:159–166. PMID: 24225429.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Loganin Prevents Hepatic Steatosis by Blocking NLRP3 Inflammasome Activation
- NLRP3 Inflammasome and Host Protection against Bacterial Infection
- The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout
- Triptolide improves myocardial fibrosis in rats through inhibition of nuclear factor kappa B and NLR family pyrin domain containing 3 inflammasome pathway
- Ethanol Augments Monosodium Urate-Induced NLRP3 Inflammasome Activation via Regulation of AhR and TXNIP in Human Macrophages