Korean Circ J.
2023 Dec;53(12):795-810. 10.4070/kcj.2023.0042.
Thalidomide and a Dipeptidyl Peptidase 4 Inhibitor in a Rat Model of Experimental Autoimmune Myocarditis
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University Medical Center, Ewha Womans University School of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2548786
- DOI: http://doi.org/10.4070/kcj.2023.0042
Abstract
- Background and Objectives
Myocarditis is a potentially fatal disease, but curative treatments have not yet been established. Myocardial inflammation is an important pathogenesis of this disease, and immunosuppressants such as methylprednisolone and immunoglobulin have been used for treatment; however, the effectiveness needs to be improved. Thalidomide and dipeptidyl peptidase (DPP) 4 inhibitors were recently investigated regarding their immunomodulatory properties. This study aimed to test whether thalidomide or a DPP4 inhibitor (evogliptin) can improve the effectiveness of myocarditis treatment using a rat model of experimental autoimmune myocarditis (EAM).
Methods
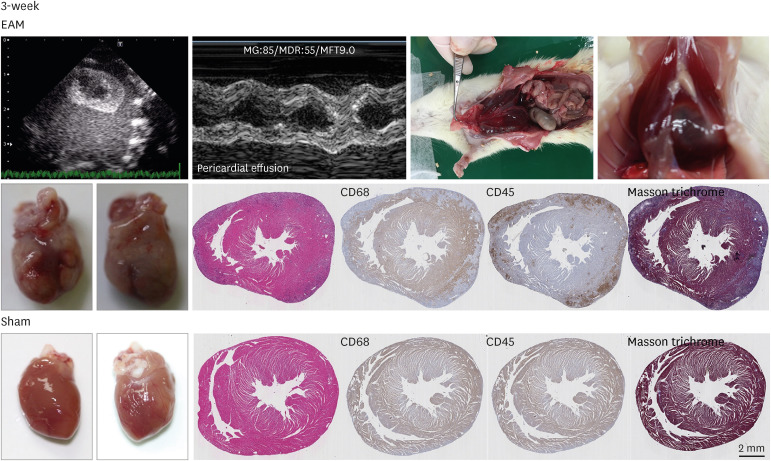
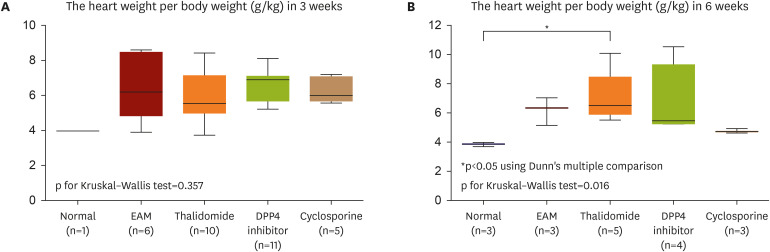
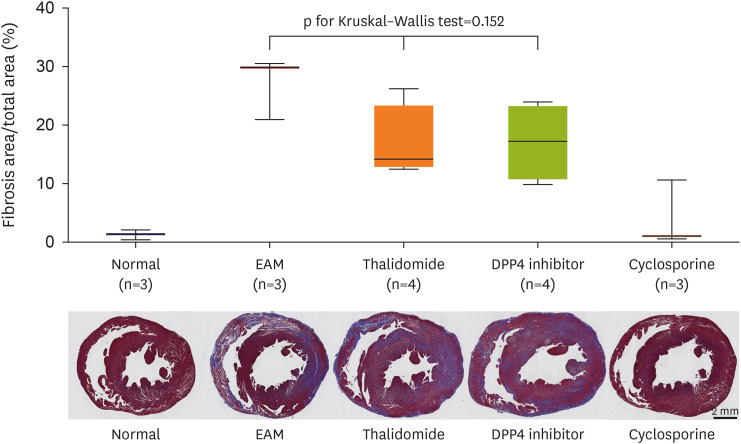
Rats with or without myocarditis were administered thalidomide at 100 mg/ kg/day and DPP4 inhibitor at 10 mg/kg/day orally. Measurement of echocardiography, serum inflammatory cytokines, myocardial histopathological examination, and immunohistochemical staining for leukocytes, macrophages, CD4+ T cells, and cytoskeleton were performed after 3 weeks, and the fibrosis area was measured after 3 and 6 weeks.
Results
Thalidomide and DPP4 inhibitor did not reduce the severity of myocarditis compared with the EAM without treatment rats by comparing the echocardiographic data, myocardial CD4 + , macrophages, neutrophil infiltrations, and the heart weight/body weight ratio in 3 weeks. The levels of inflammatory cytokines were not lower in the thalidomide and DPP4 inhibitor-treated group than in the untreated group in 3 weeks. In 6 weeks, thalidomide and DPP4 inhibitors did not reduce the fibrosis area compared to untreated groups.
Conclusions
Although thalidomide and the DPP4 inhibitor had an immunomodulatory effect and are used against inflammatory diseases, they did not ameliorate myocardial inflammation and fibrosis in this rat model of EAM.
Figure
Reference
-
1. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012; 379:738–747. PMID: 22185868.2. Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016; 68:2348–2364. PMID: 27884253.3. Chen HS, Wang W, Wu SN, Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev. 2013; 2013:CD004471. PMID: 24136037.4. Robinson J, Hartling L, Vandermeer B, Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev. 2015; 5:CD004370.5. Lv S, Yuan P, Dong J, et al. QiShenYiQi pill improves the reparative myocardial fibrosis by regulating autophagy. J Cell Mol Med. 2020; 24:11283–11293. PMID: 32881330.6. Bramuzzo M, Ventura A, Martelossi S, Lazzerini M. Thalidomide for inflammatory bowel disease: systematic review. Medicine (Baltimore). 2016; 95:e4239. PMID: 27472695.7. Chasset F, Tounsi T, Cesbron E, Barbaud A, Francès C, Arnaud L. Efficacy and tolerance profile of thalidomide in cutaneous lupus erythematosus: a systematic review and meta-analysis. J Am Acad Dermatol. 2018; 78:342–350.e4. PMID: 28989111.8. Raje N, Anderson K. Thalidomide--a revival story. N Engl J Med. 1999; 341:1606–1609. PMID: 10564693.9. Kim DH, Kim YJ, Chang SA, et al. The protective effect of thalidomide on left ventricular function in a rat model of diabetic cardiomyopathy. Eur J Heart Fail. 2010; 12:1051–1060. PMID: 20601373.10. Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009; 30:600–607. PMID: 19837468.11. Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990; 57:250–262. PMID: 2208806.12. Yuan Z, Kishimoto C, Shioji K. Beneficial effects of low-dose benidipine in acute autoimmune myocarditis: suppressive effects on inflammatory cytokines and inducible nitric oxide synthase. Circ J. 2003; 67:545–550. PMID: 12808275.13. Tang ST, Su H, Zhang Q, et al. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int J Mol Med. 2016; 37:1558–1566. PMID: 27122056.14. Zhang S, Kodama M, Hanawa H, Izumi T, Shibata A, Masani F. Effects of cyclosporine, prednisolone and aspirin on rat autoimmune giant cell myocarditis. J Am Coll Cardiol. 1993; 21:1254–1260. PMID: 8459085.15. Kodama M, Izumi T. Experimental autoimmune myocarditis. Acta Med Biol (Niigata). 1991; 39:1–10.16. Okura Y, Yamamoto T, Goto S, et al. Characterization of cytokine and iNOS mRNA expression in situ during the course of experimental autoimmune myocarditis in rats. J Mol Cell Cardiol. 1997; 29:491–502. PMID: 9140809.17. Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012; 26:2326–2335. PMID: 22552008.18. Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004; 4:314–322. PMID: 15057291.19. Yndestad A, Vinge LE, Bjørnerheim R, et al. Thalidomide attenuates the development of fibrosis during post-infarction myocardial remodelling in rats. Eur J Heart Fail. 2006; 8:790–796. PMID: 16549389.20. Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010; 327:1345–1350. PMID: 20223979.21. Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013; 54:683–687. PMID: 22966948.22. Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987; 139:3630–3636. PMID: 3680946.23. Schön E, Demuth HU, Eichmann E, et al. Dipeptidyl peptidase IV in human T lymphocytes. Impaired induction of interleukin 2 and gamma interferon due to specific inhibition of dipeptidyl peptidase IV. Scand J Immunol. 1989; 29:127–132. PMID: 2564215.24. Pitman MR, Sulda ML, Kuss B, Abbott CA. Dipeptidyl peptidase 8 and 9--guilty by association? Front Biosci (Landmark Ed). 2009; 14:3619–3633. PMID: 19273298.25. Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018; 319:1580–1591. PMID: 29677303.26. Abrahami D, Douros A, Yin H, et al. Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study. BMJ. 2018; 360:k872. PMID: 29563098.27. Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011; 55:10–16. PMID: 21664294.28. Hirakawa H, Zempo H, Ogawa M, et al. A DPP-4 inhibitor suppresses fibrosis and inflammation on experimental autoimmune myocarditis in mice. PLoS One. 2015; 10:e0119360. PMID: 25768281.29. Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005; 54:146–151. PMID: 15616022.30. Timmers L, Henriques JP, de Kleijn DP, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009; 53:501–510. PMID: 19195607.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Emerging Safety Issues of Dipeptidyl Peptidase-4 Inhibitors and Sodium Glucose Cotransporter 2 Inhibitors: How to Interpret and Apply in Clinical Practice
- Letter: Association between Serum Dipeptidyl Peptidase-4 Concentration and Obesity-Related Factors in Health Screen Examinees (J Obes Metab Syndr 2017;26:188-96)
- Novel Therapies for Type 2 Diabetes Mellitus
- Comparison of DPP-4 Inhibitors