J Korean Med Sci.
2008 Jun;23(3):492-501. 10.3346/jkms.2008.23.3.492.
The Role of Cyclosporine and Mycophenolate in an Orthotopic Porcine-to-Rat Corneal Xenotransplantation
- Affiliations
-
- 1Seoul Artificial Eye Center, Seoul National University Hospital Clinical Research Institute, Seoul, Korea.
- 2Department of Ophthalmology, College of Medicine, Chung-Ang University, Seoul, Korea.
- 3Department of Ophthalmology, College of Medicine, Seoul National University, Seoul, Korea. kmk9@snu.ac.kr
- KMID: 1786890
- DOI: http://doi.org/10.3346/jkms.2008.23.3.492
Abstract
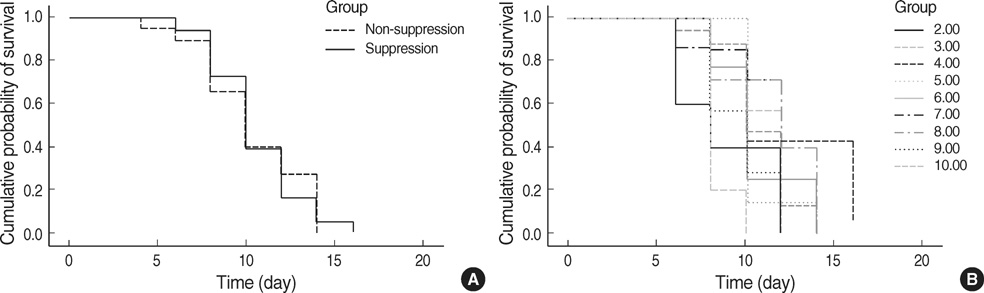
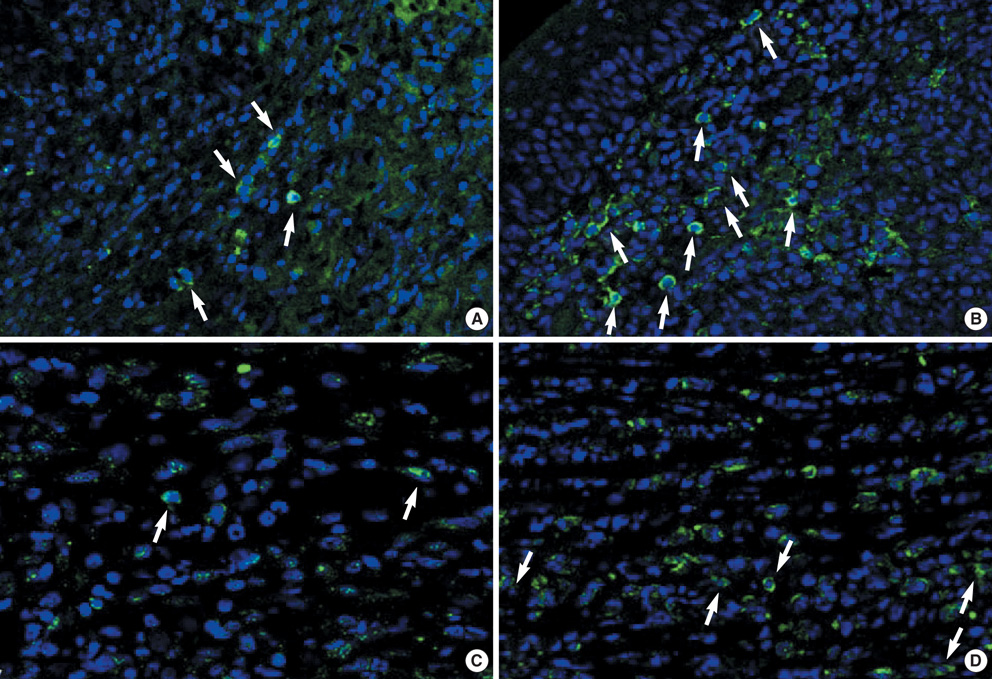
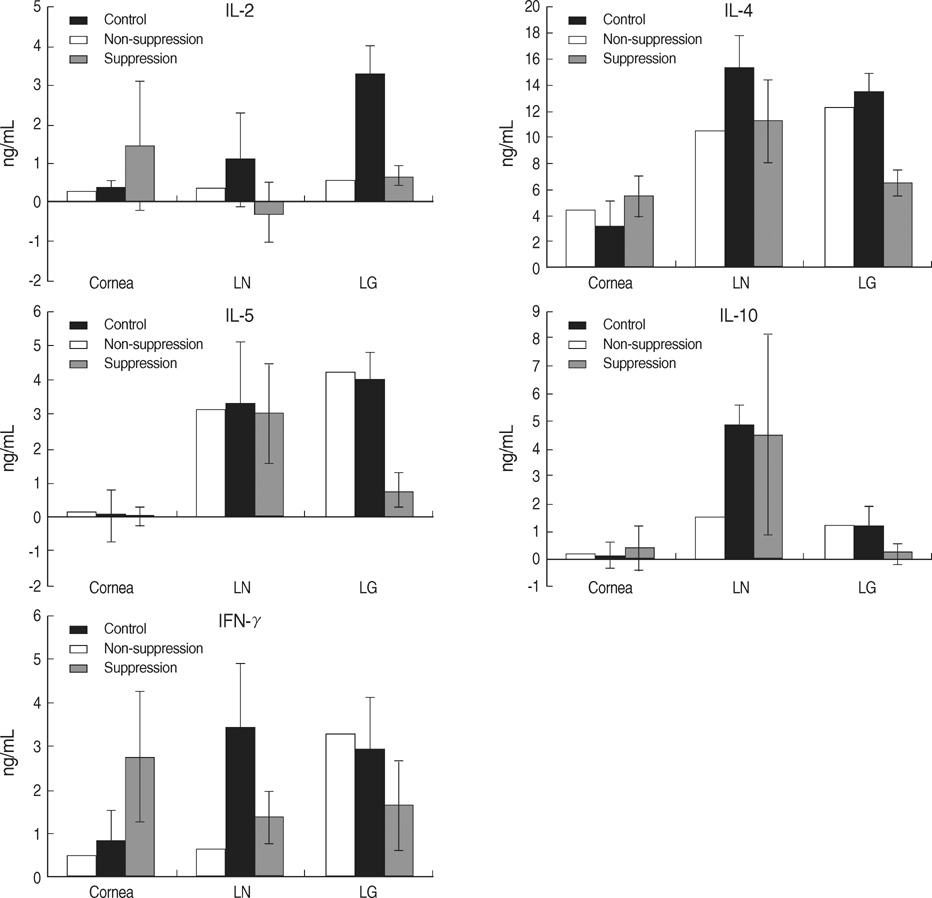
- We performed this study to investigate the feature of rejection in porcine-to-rat corneal orthotopic transplantation and to evaluate the effect of cyclosporine and mycophenolate on the xeno-rejection. Orthotopic corneal transplantation was done at 91 Sprague-Dawley rats, and they were divided into 10 groups based on the combination of immunosuppressants including dexamethasone, cyclosporine, and mycophenolate mofetil. Graft survival was analyzed and grafted eyes were examined with Hematoxylin & Eosin and CD4 or CD8 staining. Enzyme-linked immunosorbent assays were done for interleukin-2 (IL-2), IL-4, IL-5, IL-10, and interferon (IFN)-gamma in cornea, lacrimal gland, and cervical lymph nodes. The longest median survival of the immune suppressant group was 11.00+/-1.96 days, which showed no statistical differences compared with that of control (8.00+/-1.52 days). The neutrophils were prominent in the early phase but soon gave way to the monocytes. The number of CD8+ cells was higher than that of CD4+ cells. IL-2 and IFN-gamma markedly increased at 10 to13 days in cornea, lacrimal glands, and cervical lymph nodes, which showed a decrease with immunosuppressants except in the cornea. In conclusion, cyclosporine and mycophenolate could not prevent the rejection in porcine to rat orthotopic corneal xenograft associated with infiltraton of CD8+ and innate immune cells.
Keyword
MeSH Terms
-
Animals
*Corneal Transplantation
Cyclosporine/*pharmacology
Cytokines/metabolism
Graft Rejection/immunology/pathology/*prevention & control
Graft Survival/*drug effects
Immunosuppressive Agents/*pharmacology
Interferon-gamma/metabolism
Interleukin-10/metabolism
Interleukin-2/metabolism
Interleukin-4/metabolism
Interleukin-5/metabolism
Mycophenolic Acid/*analogs & derivatives/pharmacology
Neutrophils/immunology
Rats
Rats, Sprague-Dawley
Swine
Transplantation, Heterologous
Figure
Reference
-
1. Amano S, Shimomura N, Kaji Y, Ishii K, Yamagami S, Araie M. Antigenicity of porcine cornea as xenograft. Curr Eye Res. 2003. 26:313–318.
Article2. Sano Y, Okamoto S, Streilein JW. Induction of donor-specific ACAID can prolong orthotopic corneal allograft survival in "high-risk" eyes. Curr Eye Res. 1997. 16:1171–1174.3. Tanaka K, Yamada J, Joyce N, Streilein JW. Immunobiology of xenogeneic cornea grafts in mouse eyes. I. Fate of xenogeneic cornea tissue grafts implanted in anterior chamber of mouse eyes. Transplantation. 2000. 69:610–616.4. Tanaka K, Yamada J, Streilein JW. Xenoreactive CD4+ T cells and acute rejection of orthotopic guinea pig corneas in mice. Invest Ophthalmol Vis Sci. 2000. 41:1827–1832.5. Yamada J, Kurimoto I, Streilein JW. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sc. 1999. 40:2614–2621.6. Sonoda KH, Nakao S, Nakamura T, Oshima T, Qiao H, Hisatomi T, Kinoshita S, Ishibashi T. Cellular events in the normal and inflamed cornea. Cornea. 2005. 24:Suppl 8. S50–S54.
Article7. Larkin DF, Takano T, Standfield SD, Williams KA. Experimental orthotopic corneal xenotransplantation in the rat. Mechanisms of graft rejection. Transplantation. 1995. 60:491–497.
Article8. Rood PP, Buhler LH, Bottino R, Trucco M, Cooper DK. Pig-to-non-human primate islet xenotransplantation: a review of current problems. Cell Transplant. 2006. 15:89–104.
Article9. Cabezuelo JB, Ramirez P, Chavez R, Majado M, Munitiz V, Munoz A, Hernandez Q, G-Palenciano C, Pino-Chavez G, Loba M, Yelamos J, Vizcaino AS, Cayuela M, Segura B, Marin F, Rubio A, Fuente T, Gago MR, Rios A, Montoya M, Esteban A, Bueno FS, Robles R, Cozzi E, White DJ, Parrilla P. Assessment of renal function during the postoperative period following liver xenotransplantation from transgenic pig to baboon. Transplant Proc. 2002. 34:321–322.
Article10. Candinas D, Belliveau S, Koyamada N, Miyatake T, Hechenleitner P, Mark W, Bach FH, Hancock WW. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection. Transplantation. 1996. 62:1920–1927.11. Tanemura M, Galili U. T cells interacting with the alpha-Gal epitope: studies in alpha1, 3 Galactosyltransferase knock-out mice. Transplant Proc. 2000. 32:921–923.12. McKenzie IF, Koulmanda M, Mandel TE, Sandrin MS. Pig islet xenografts are susceptible to "anti-pig" but not Gal alpha(1,3)Gal antibody plus complement in Gal o/o mice. J Immunol. 1998. 161:5116–5119.13. Rijkelijkhuizen JK, Haanstra KG, Wubben J, Tons A, Roos A, van Gijlswijk-Janssen DJ, Ringers J, Bouwman E, Jonker M. T-cell-specific immunosuppression results in more than 53 days survival of porcine islets of langerhans in the monkey. Transplantation. 2003. 76:1359–1368.
Article14. Qian Y, Dana MR. Molecular mechanisms of immunity in corneal allotransplantation and xenotransplantation. Expert Rev Mol Med. 2001. 3:1–21.
Article15. Kobashigawa JA, Miller LW, Russell SD, Ewald GA, Zucker MJ, Goldberg LR, Eisen HJ, Salm K, Tolzman D, Gao J, Fitzsimmons W, First R. Study Investigators. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006. 6:1377–1386.
Article16. Simeonovic CJ, Ceredig R, Wilson JD. Effect of GK1.5 monoclonal antibody dosage on survival of pig proislet xenografts in CD4+ T cell-depleted mice. Transplantation. 1990. 49:849–856.
Article17. Karlsson-Parra A, Ridderstad A, Wallgren AC, Moller E, Ljunggren HG, Korsgren O. Xenograft rejection of porcine islet-like cell clusters in normal and natural killer cell-depleted mice. Transplantation. 1996. 61:1313–1320.18. Nielsen B, Steinbruchel DA, Lillevang ST, Kemp E. Evidence for a primarily humoral rejection mechanism in concordant xenogeneic heart transplantation. A sequential immunohistological study in a hamster-to-rat model. APMIS. 1993. 101:587–594.
Article19. Brouard S, Gagne K, Blancho G, Soulillou JP. T cell response in xenorecognition and xenografts: a review. Hum Immunol. 1999. 60:455–468.
Article20. Goslings WR, Yamada J, Dana MR, Streilein JW, van Beelen E, Prodeus AP, Carroll MC, Jager MJ. Corneal transplantation in antibody-deficient hosts. Invest Ophthalmol Vis Sci. 1999. 40:250–253.21. Andres A, Toso C, Morel P, Bosco D, Bucher P, Oberholzer J, Mathe Z, Mai G, Wekerle T, Berney T, Buhler LH. Macrophage depletion prolongs discordant but not concordant islet xenograft survival. Transplantation. 2005. 79:543–549.
Article22. Tanaka K, Sonoda K, Streilein JW. Acute rejection of orthotopic corneal xenografts in mice depends on CD4(+) T cells and self-antigen-presenting cells. Invest Ophthalmol Vis Sci. 2001. 42:2878–2884.23. Laumonier T, Walpen AJ, Maurus CF, Mohacsi PJ, Matozan KM, Korchagina EY, Bovin NV, Vanhove B, Seebach JD, Rieben R. Dextran sulfate acts as an endothelial cell protectant and inhibits human complement and natural killer cell-mediated cytotoxicity against porcine cells. Transplantation. 2003. 76:838–843.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA Microarray-Based Gene Expression Profiling in Porcine Keratocytes and Corneal Endothelial Cells and Comparative Analysis Associated with Xeno-related Rejection
- Current status of pancreatic islet xenotransplantation
- Experimental Model of Cardiac Xenograft , Mouse Heart to Rat
- Overcoming Immunological Barriers in Xenotransplantation: A Historical Review of Previous Research and Future Directions
- Experimental orthotopic penetrating keratoplasty--a rat penetrating keratoplasty model