J Korean Med Sci.
2009 Apr;24(2):189-196. 10.3346/jkms.2009.24.2.189.
DNA Microarray-Based Gene Expression Profiling in Porcine Keratocytes and Corneal Endothelial Cells and Comparative Analysis Associated with Xeno-related Rejection
- Affiliations
-
- 1Seoul Artificial Eye Center, Seoul National University Hospital Clinical Research Institute, Seoul, Korea. wrwee@snu.ac.kr
- 2Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea.
- 3Xenotransplantation Research Center and Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 7Division of Molecular and Life Science, Hanyang University, Seoul, Korea.
- 8GenoCheck Co. Ltd., Ansan, Korea.
- KMID: 1779117
- DOI: http://doi.org/10.3346/jkms.2009.24.2.189
Abstract
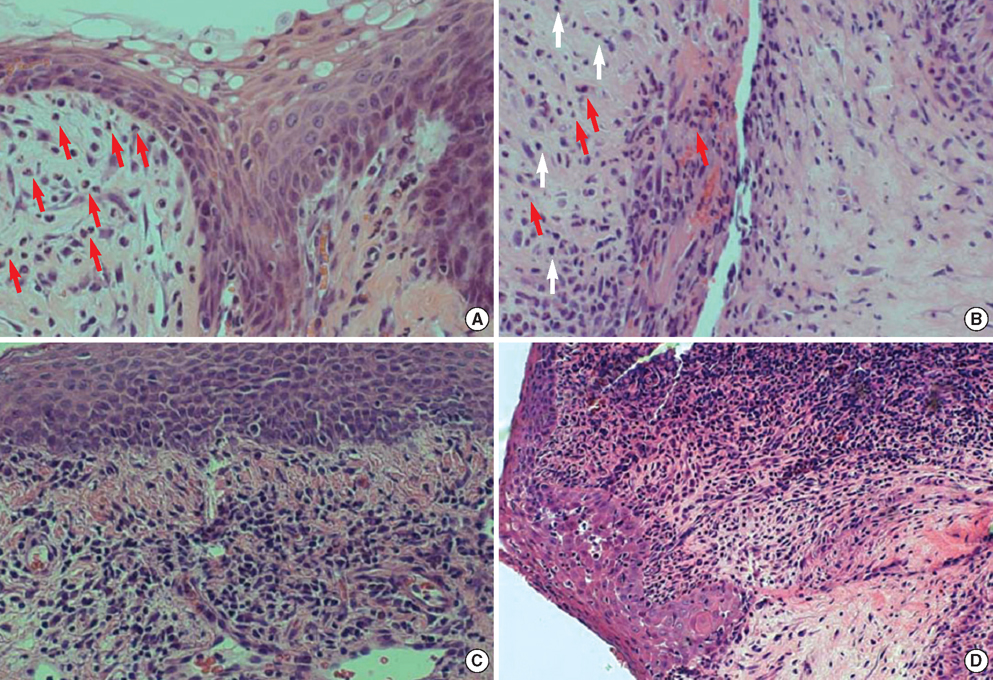
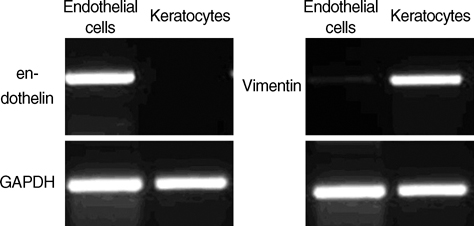
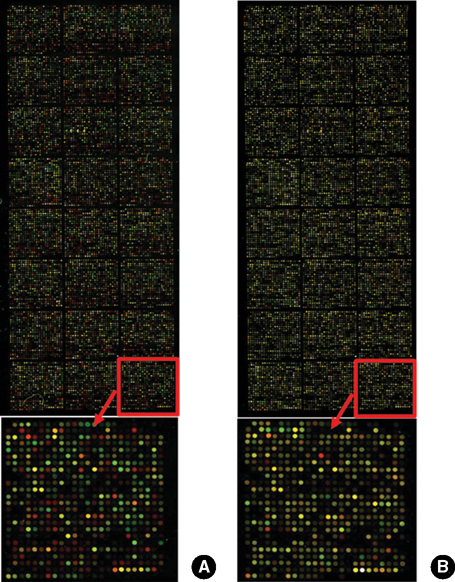
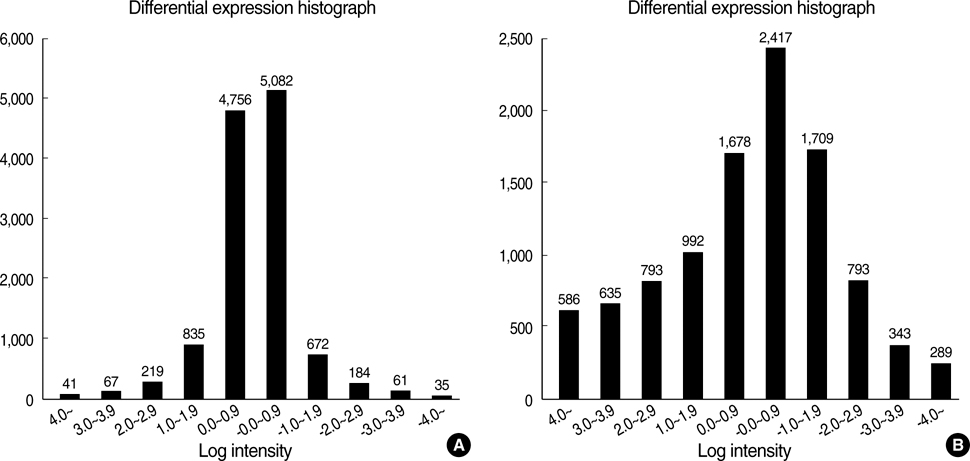
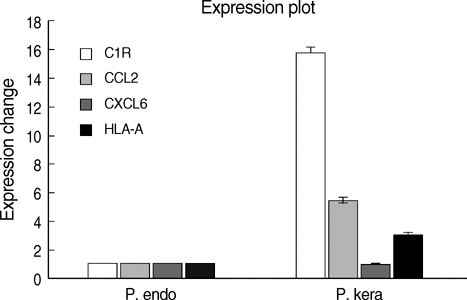
- Porcine to rat corneal xenotransplantation resulted in severe inflammation and rejection of the corneal stroma, whereas an allograft showed mainly endothelial cell-associated rejection. We, therefore, investigated and compared the gene expression between porcine keratocytes and corneal endothelial cells. RNA was isolated from primary cultured porcine or human keratocytes and porcine corneal endothelial cells. Gene expression was comparatively analyzed after normalization with microarray method using Platinum pig 13 K oligo chip (GenoCheck Co., Ltd., Ansan, Korea). Real-time polymerase chain reaction (PCR) was performed for C1R, CCL2, CXCL6, and HLA-A in porcine keratocytes and corneal endothelial cells. As a result, upregulated expression more than 2 folds was observed in 1,162 genes of porcine keratocytes versus porcine endothelial cells. Among the immune-regulatory genes, SEMA3C, CCL2, CXCL6, F3, HLA-A, CD97, IFI30, C1R, and G1P3 were highly expressed in porcine keratocytes, compared to porcine corneal endothelial cells or human keratocytes. When measured by real-time PCR, the expression of C1R, CCL2, and HLA-A was higher in porcine keratocytes compared to that in porcine corneal endothelial cells. In conclusion, the increased expression of C1R, CCL2, and HLA-A genes in porcine keratocytes might be responsible for the stromal rejection observed in a porcine to rat corneal xenotransplantation.
Keyword
MeSH Terms
-
Animals
Cells, Cultured
Chemokine CCL2/metabolism
Complement C1r/metabolism
Corneal Transplantation/*immunology/pathology
Endothelium, Corneal/*metabolism/pathology
*Gene Expression Profiling
Graft Rejection/*immunology/pathology
HLA-A Antigens/metabolism
Humans
Keratinocytes/*metabolism
Oligonucleotide Array Sequence Analysis
Rats
Reverse Transcriptase Polymerase Chain Reaction
Swine
Transplantation, Heterologous
Up-Regulation
Figure
Reference
-
1. Theobald VA, Lauer JD, Kaplan FA, Baker KB, Rosenberg M. "Neutral allografts"--lack of allogeneic stimulation by cultured human cells expressing MHC class I and class II antigens. Transplantation. 1993. 55:128–133.
Article2. Foulks GN. Krachmer JH, Mannis MJ, Holland EJ, editors. Diagnosis and management of corneal allograft rejection. Cornea. 2005. Philadelphia: Elsevier Mosby;1541–1549.
Article3. Zhu X, Dor FJ, Cooper DK. Pig-to-non-human primate heart transplantation: immunologic progress over 20 years. J Heart Lung Transplant. 2007. 26:210–218.
Article4. Cooper DK. Rene Kuss's clinical experience with pig kidney transplantation in 1966. Xenotransplantation. 2007. 14:93.
Article5. Rood PP, Buhler LH, Bottino R, Trucco M, Cooper DK. Pig-to-nonhuman primate islet xenotransplantation: a review of current problems. Cell Transplant. 2006. 15:89–104.
Article6. Amano S, Shimomura N, Kaji Y, Ishii K, Yamagami S, Araie M. Antigenicity of porcine cornea as xenograft. Curr Eye Res. 2003. 26:313–318.
Article7. Kim MK, Oh JY, Lee HI, Ko JH, Lee HJ, Lee JH, Wee WR. Susceptibility of porcine keratocytes to immune-mediated damage in xeno-related rejection. Transplant Proc. 2008. 40:564–569.
Article8. Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995. 270:467–470.
Article9. Zhao SH, Recknor J, Lunney JK, Nettleton D, Kuhar D, Orley S, Tuggle CK. Validation of a first-generation long-oligonucleotide microarray for transcriptional profiling in the pig. Genomics. 2005. 86:618–625.
Article10. Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002. 30:e15.
Article11. Kerényi A, Nagy G, Veres A, Varga L, Füst A, Nagymihány A, Czumbel N, Süveges I, Füst G. C1r-C1s-C1inhibitor (C1rs-C1inh) complex measurements in tears of patients before and after penetrating keratoplasty. Curr Eye Res. 2002. 24:99–104.
Article12. Bordet J. Mak TW, Saunders ME, editors. Complement. The immune response. 2006. San Diego: Elsevier;553–581.13. Mondino BJ, Sundar-Raj CV, Brady KJ. Production of first component of complement by corneal fibroblasts in tissue culture. Arch Ophthalmol. 1982. 100:478–480.
Article14. Ohno K, Mitooka K, Nelson LR, Hodge DO, Bourne WM. Keratocyte activation and apoptosis in transplanted human corneas in a xenograft model. Invest Ophthalmol Vis Sci. 2002. 43:1025–1031.15. Paule MF, McColl SR, Simeonovic CJ. Murine chemokine gene expression in rejecting pig proislet xenografts. Transplant Proc. 2000. 32:1062.
Article16. Solomon MF, Kuziel WA, Mann DA, Simeonovic CJ. The role of chemokines and their receptors in the rejection of pig islet tissue xenografts. Xenotransplantation. 2003. 10:164–177.
Article17. Ehrnfelt C, Kumagai-Braesch M, Uzunel M, Holgersson J. Adult porcine islets produce MCP-1 and recruit human monocytes in vitro. Xenotransplantation. 2004. 11:184–194.
Article18. Zhang T, Suzuki J, Kawauchi M, Nakano H, Kuroda H, Koide N, Kitahara H, Ohta K, Wada Y, Tsukioka K, Takayama K, Endoh M, Takamoto S, Isobe M, Amano J. Expression of monocyte chemoattractant protein 1 in pig-to-primate cardiac xenografts. Transplant Proc. 2000. 32:984–986.
Article19. Struyf S, Gouwy M, Dillen C, Proost P, Opdenakker G, Van Damme J. Chemokines synergize in the recruitment of circulating neutrophils into inflamed tissue. Eur J Immunol. 2005. 35:1583–1591.
Article20. Chen D, Weber M, Mcvey JH, Kemball-Cook G, Tuddenham EG, Lechler RI, Dorling A. Complete inhibition of acute humoral rejection using regulated expression of membrane-tethered anticoagulants on xenograft endothelium. Am J Transplant. 2004. 4:1958–1963.
Article21. Cozzi E, Simioni P, Boldrin M, Seveso M, Calabrese F, Baldan N, Busetto R, Tormene D, Gavasso S, Castagnaro M, Echelard Y, Rice T, Plebani M, Carraro P, Bosio E, Valente M, Pagnan A, Thiene G, Ancona E. Effects of long-term administration of high-dose recombinant human antithrombin in immunosuppressed primate recipients of porcine xenografts. Transplantation. 2005. 80:1501–1510.
Article22. Wang T, Tian L, Haino M, Gao JL, Lake R, Ward Y, Wang H, Siebenlist U, Murphy PM, Kelly K. Improved antibacterial host defense and altered peripheral granulocyte homeostasis in mice lacking the adhesion class G protein receptor CD97. Infect Immun. 2007. 75:1144–1153.
Article23. Barjaktarevic I, Rahman A, Radoja S, Bogunović B, Vollmer A, Vukmanović S, Marić M. Inhibitory role of IFN-gamma-inducible lysosomal thiol reductase in T cell activation. J Immunol. 2006. 177:4369–4375.24. Tahara E, Tahara H, Kanno M, Naka K, Takeda Y, Matsuzaki T, Yamazaki R, Ishihara H, Yasui W, Barrett JC, Ide T, Tahara E. G1P3, an interferon inducible gene 6-16, is expressed in gastric cancers and inhibits mitochondrial-mediated apoptosis in gastric cancer cell line TMK-1 cell. Cancer Immunol Immunother. 2005. 54:729–730.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gene Microarray Related with Apoptosis in Diabetic OLETF Keratocytes
- Comparison of oligonucleotide-microarray and serial analysis of gene expression (SAGE) in transcript profiling analysis of megakaryocytes derived from CD34+ cells
- A Case of Korean Patient with Macular Corneal Dystrophy Associated with Novel Mutation in the CHST6 Gene
- Gene Expression Profiling of Meningioma by cDNA Chip
- Microarray for Genes Associated with Signal Transduction in Diabetic OLETF Keratocytes