Yonsei Med J.
2006 Dec;47(6):862-869. 10.3349/ymj.2006.47.6.862.
Toxoplasma gondi Inhibits Apoptosis in Infected Cells by Caspase Inactivation and NF-kappaB Activation
- Affiliations
-
- 1Department of Parasitology, Institute of Biomedical Science, Hanyang University College of Medicine, Seoul, Korea. mhahn@hanyang.ac.kr
- KMID: 1777177
- DOI: http://doi.org/10.3349/ymj.2006.47.6.862
Abstract
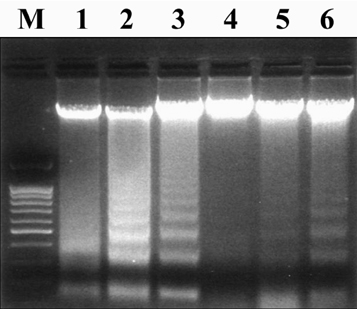
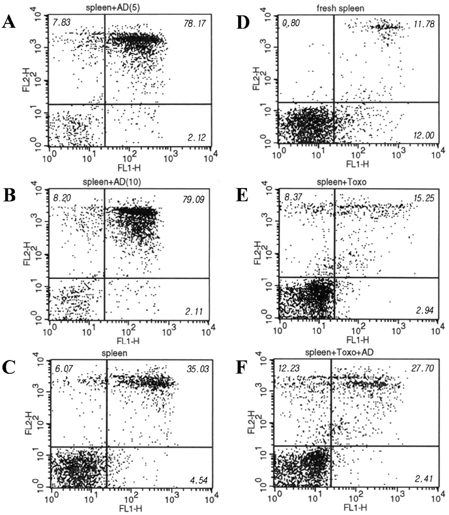
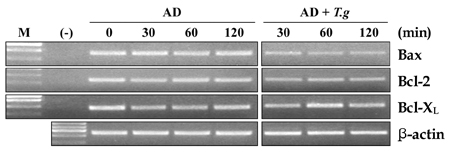
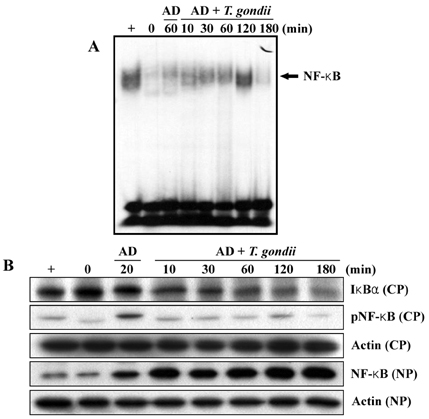
- Our experiments aimed to clarify the mechanism by which host cell apoptosis is inhibited by infection with the intracellular protozoan parasite, Toxoplasma gondii (T. gondii). Mouse spleen cells were cultured in 6-well plates with RPMI 1640/ 10% FBS at 37(i)E, in a 5% CO2 atmosphere. Apoptosis of spleen cells was induced by actinomycin-D (AD) treatment for 1 h prior to infection with T. gondii. A variety of assays were used to assess the progression of apoptosis: DNA size analysis on agarose gel electrophoresis, flow cytometry with annexin V/PI staining, and analysis of expression levels of Bcl-2 family and NF-kappaB mRNA and proteins by RT-PCR, Western blotting, and EMSA. Additionally, transmission electron microscopy (TEM) was performed to observe changes in cell morphology. Fragmentation of DNA was inhibited in spleen cells treated with AD and T. gondii 5 h and 18 h post infection, respectively, and flow cytometry studies showed a decreased apoptotic rates in AD and T. gondii treated spleen cells. We observed decreased expression of Bax mRNA and protein, while levels of Bcl-2 mRNA remained constant in spleen cells treated with AD and T. gondii. Caspase 3 and PARP were inactivated in cells treated with AD and T. gondii, and increased levels of cleaved caspase 8 were also observed. Analysis of EMSA and Western blot data suggests that activation of transcription factor NF-kappaB may be involved in the blockade of apoptosis by T. gondii. TEM analysis showed nuclear fragmentation and chromatin condensation occurring in spleen cells treated with AD; however, such apoptosis- associated morphological changes were not observed in cells treated with both AD and T. gondii tachyzoites. Together, these data show that T. gondii infection inhibits AD induced apoptosis via caspase inactivation and NF-kappaB activation in mouse spleen cells.
MeSH Terms
Figure
Reference
-
1. Payne JM, Molestina RE, Sinai AP. Inhibition of caspase activation and requirement for NF-κB function in the Toxoplasma gondii-mediated blockade of host apoptosis. J Cell Sci. 2003. 116:4345–4358.2. Shin HJ, Cho MS, Kim HI, Lee M, Park S, Sohn S, et al. Apoptosis of primary-culture rat microglial cells induced by pathogenic Acanthamoeba spp. Clin Diagn Lab Immunol. 2000. 7:510–514.3. Sim S, Kim KA, Yong TS, Park SJ, Im KI, Shin MH. Ultrastructural observation of human neutrophils during apoptotic cell death triggered by Entamoeba histolytica. Korean J Parasitol. 2004. 42:205–208.4. Sim S, Yong TS, Park SJ, Im KI, Kong Y, Ryu JS, et al. NADPH oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica. J Immunol. 2005. 174:4279–4288.5. Liesenfeld O, Kosek JC, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun. 1997. 65:4682–4689.6. Hu MS, Schwartzman JD, Yeaman GR, Collins J, Seguin R, Khan IA, et al. Fas-FasL interaction involved in pathogenesis of ocular toxoplasmosis in mice. Infect Immun. 1999. 67:928–935.7. Nishikawa Y, Makala L, Otsuka H, Mikami T, Nagasawa H. Mechanisms of apoptosis in murine fibroblasts by two intracellular protozoan parasites, Toxoplasma gondii and Neospora caninum. Parasite Immunol. 2002. 24:347–354.8. Gavrilescu LC, Denkers EY. IFN-γ overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J Immunol. 2001. 167:902–909.9. Nash PB, Purner MB, Leon RP, Clarke P, Duke RC, Curiel TJ. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J Immunol. 1998. 160:1824–1830.10. Kim JS, Kim JM, Jung HC, Song IS. Caspase-3 activity and expression of Bcl-2 family in human neutrophils by Helicobacter pylori water-soluble proteins. Helicobacter. 2001. 6:207–215.11. Elewaut D, DiDonato JA, Kim JM, Truong F, Eckmann L, Kagnoff MF. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J Immunol. 1999. 163:1457–1466.12. Kim JM, Oh YK, Kim YJ, Cho SJ, Ahn MH, Cho YJ. Nuclear factor-kappa B plays a major role in the regulation of chemokine expression of HeLa cells in response to Toxoplasma gondii infection. Parasitol Res. 2001. 87:758–763.13. Chang JH, Ryang YS, Morio T, Lee SK, Chang EJ. Trichomonas vaginalis inhibits proinflammatory cytokine production in macrophages by suppressing NF-κB activation. Mol Cells. 2004. 18:177–185.14. Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthoft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998. 102:1815–1823.15. Goebel S, Luder CG, Gross U. Invasion by Toxoplasma gondii protects human-derived HL-60 cells from actinomycin D-induced apoptosis. Med Microbiol Immunol. 1999. 187:221–226.16. Goebel S, Gross U, Luder CG. Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly (ADP-ribose) polymerase expression. J Cell Sci. 2001. 114:3495–3505.17. Heussler VT, Kuenzi P, Rottenberg S. Inhibition of apoptosis by intracellular protozoan parasites. Int J Parasitol. 2001. 31:1166–1176.18. Molestina RE, Payne TM, Coppens I, Sinai AP. Activation of NF-kappaB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IkappaB to the parasitophorous vacuole membrane. J Cell Sci. 2003. 116:4359–4371.19. Denkers EY, Butcher BA, Del Rio L, Kim L. Manipulation of mitogen-activated protein kinase/nuclear factor-kappa B-signaling cascades during intracellular Toxoplasma gondii infection. Immunol Rev. 2004. 201:191–205.20. Carmen JC, Hardi L, Sinai AP. Toxoplasma gondii inhibits ultraviolet light-induced apoptosis through multiple interactions with the mitochondrion-dependent programmed cell death pathway. Cell Microbiol. 2006. 8:301–315.21. Sinai AP, Payne TM, Carmen JC, Hardi L, Watson SJ, Molestina RE. Mechanisms underlying the manipulation of host apoptotic pathways by Toxoplasma gondii. Int J Parasitol. 2004. 34:381–391.22. James ER, Green DR. Manipulation of apoptosis in the host-parasite interaction. Trends Parasitol. 2004. 20:280–287.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PDTC Inhibits TNF-alpha-Induced Apoptosis in MC3T3E1 Cells
- Caspase-3 Activation Leads to Apoptosis of Human Gastric Epithelial Cells Infected with Helicobacter pylori
- Balsalazide Potentiates Parthenolide-Mediated Inhibition of Nuclear Factor-kappaB Signaling in HCT116 Human Colorectal Cancer Cells
- The Role of NF-kappaB in the TNF-alpha-induced Apoptosis of Lung Cancer Cell Line
- NF-kappaB Activation in T Helper 17 Cell Differentiation