Korean J Physiol Pharmacol.
2012 Jun;16(3):181-186. 10.4196/kjpp.2012.16.3.181.
Reduction of Food Intake by Fenofibrate is Associated with Cholecystokinin Release in Long-Evans Tokushima Rats
- Affiliations
-
- 1Department of Internal Medicine, Medical Science Research Center, Mitochondria Hub Regulation Center, Dong-A University College of Medicine, Busan 602-714, Korea.
- 2Department of Pharmacology, Medical Science Research Center, Mitochondria Hub Regulation Center, Dong-A University College of Medicine, Busan 602-714, Korea. hjlee@dau.ac.kr
- KMID: 1768011
- DOI: http://doi.org/10.4196/kjpp.2012.16.3.181
Abstract
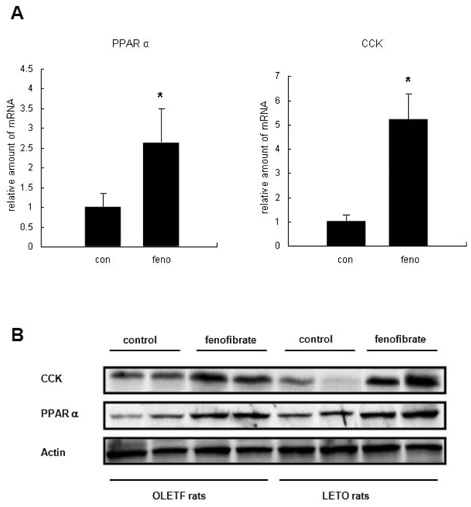
- Fenofibrate is a selective peroxisome proliferator-activated receptor alpha (PPARalpha) activator and is prescribed to treat hyperlipidemia. The mechanism through which PPARalpha agonists reduce food intake, body weight, and adiposity remains unclear. One explanation for the reduction of food intake is that fenofibrate promotes fatty acid oxidation and increases the production of ketone bodies upon a standard experimental dose of the drug (100~300 mg/kg/day). We observed that low-dose treatment of fenofibrate (30 mg/kg/day), which does not cause significant changes in ketone body synthesis, reduced food intake in Long-Evans Tokushima (LETO) rats. LETO rats are the physiologically normal controls for Otsuka Long-Evans Tokushima Fatty (OLETF) rats, which are obese and cholecystokinin (CCK)-A receptor deficient. We hypothesized that the reduced food intake by fenofibrate-treated LETO rats may be associated with CCK production. To investigate the anorexic effects of fenofibrate in vivo and to determine whether CCK production may be involved, we examined the amount of food intake and CCK production. Fenofibrate-treated OLETF rats did not significantly change their food intake while LETO rats decreased their food intake. Treatment of fenofibrate increased CCK synthesis in the duodenal epithelial cells of both LETO and OLETF rats. The absence of a change in the food intake of OLETF rats, despite the increase in CCK production, may be explained by the absence of CCK-A receptors. Contrary to the OLETF rats, LETO rats, which have normal CCK receptors, presented a decrease in food intake and an increase in CCK production. These results suggest that reduced food intake by fenofibrate treatment may be associated with CCK production.
Keyword
MeSH Terms
-
Adiposity
Animals
Body Weight
Cholecystokinin
Diethylpropion
Eating
Epithelial Cells
Fenofibrate
Hyperlipidemias
Ketone Bodies
PPAR alpha
Rats
Rats, Inbred OLETF
Receptor, Cholecystokinin A
Receptors, Cholecystokinin
Cholecystokinin
Diethylpropion
Fenofibrate
Ketone Bodies
PPAR alpha
Receptor, Cholecystokinin A
Receptors, Cholecystokinin
Figure
Reference
-
1. Fazio S, Linton MF. The role of fibrates in managing hyperlipidemia: mechanisms of action and clinical efficacy. Curr Atheroscler Rep. 2004. 6:148–157.2. Larsen PJ, Jensen PB, Sørensen RV, Larsen LK, Vrang N, Wulff EM, Wassermann K. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes. 2003. 52:2249–2259.3. Lee HJ, Choi SS, Park MK, An YJ, Seo SY, Kim MC, Hong SH, Hwang TH, Kang DY, Garber AJ, Kim DK. Fenofibrate lowers abdominal and skeletal adiposity and improves insulin sensitivity in OLETF rats. Biochem Biophys Res Commun. 2002. 296:293–299.4. Park CW, Zhang Y, Zhang X, Wu J, Chen L, Cha DR, Su D, Hwang MT, Fan X, Davis L, Striker G, Zheng F, Breyer M, Guan Y. PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 2006. 69:1511–1517.5. Srivastava RA, Jahagirdar R, Azhar S, Sharma S, Bisgaier CL. Peroxisome proliferator-activated receptor-alpha selective ligand reduces adiposity, improves insulin sensitivity and inhibits atherosclerosis in LDL receptor-deficient mice. Mol Cell Biochem. 2006. 285:35–50.6. Yamamoto K, Fukuda N, Zhang L, Sakai T. Altered hepatic metabolism of fatty acids in rats fed a hypolipidaemic drug, fenofibrate. Pharmacol Res. 1996. 33:337–342.7. Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001. 21:193–230.8. Fisler JS, Egawa M, Bray GA. Peripheral 3-hydroxybutyrate and food intake in a model of dietary-fat induced obesity: effect of vagotomy. Physiol Behav. 1995. 58:1–7.9. Langhans W, Pantel K, Scharrer E. Ketone kinetics and D-(-)-3-hydroxybutyrate-induced inhibition of feeding in rats. Physiol Behav. 1985. 34:579–582.10. Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol. 1988. 255:R974–R981.11. Bhavsar S, Watkins J, Young A. Synergy between amylin and cholecystokinin for inhibition of food intake in mice. Physiol Behav. 1998. 64:557–561.12. Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obes Rev. 2005. 6:297–306.13. Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol. 2004. 55:137–154.14. Funakoshi A, Miyasaka K, Shinozaki H, Masuda M, Kawanami T, Takata Y, Kono A. An animal model of congenital defect of gene expression of cholecystokinin (CCK)-A receptor. Biochem Biophys Res Commun. 1995. 210:787–796.15. Lee JH, Kim CH, Kim DG, Ahn YS. Microarray Analysis of Differentially Expressed Genes in the Brains of Tubby Mice. Korean J Physiol Pharmacol. 2009. 13:91–97.16. Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol. 1998. 274:R618–R625.17. Sanders MA, Basson MD. Collagen IV-dependent ERK activation in human Caco-2 intestinal epithelial cells requires focal adhesion kinase. J Biol Chem. 2000. 275:38040–38047.18. Carmona MC, Louche K, Nibbelink M, Prunet B, Bross A, Desbazeille M, Dacquet C, Renard P, Casteilla L, Pénicaud L. Fenofibrate prevents Rosiglitazone-induced body weight gain in ob/ob mice. Int J Obes (Lond). 2005. 29:864–871.19. Nishimura J, Dewa Y, Muguruma M, Kuroiwa Y, Yasuno H, Shima T, Jin M, Takahashi M, Umemura T, Mitsumori K. Effect of fenofibrate on oxidative DNA damage and on gene expression related to cell proliferation and apoptosis in rats. Toxicol Sci. 2007. 97:44–54.20. Bünger M, van den Bosch HM, van der Meijde J, Kersten S, Hooiveld GJ, Müller M. Genome-wide analysis of PPARalpha activation in murine small intestine. Physiol Genomics. 2007. 30:192–204.21. Ferreira AV, Parreira GG, Green A, Botion LM. Effects of fenofibrate on lipid metabolism in adipose tissue of rats. Metabolism. 2006. 55:731–735.22. Han Y, Do MH, Kim MS, Seo E, Park MK, Kim DK, Lee HJ, Seo SY. Fenofibrate Reduces Age-related Hypercholesterolemia in Normal Rats on a Standard Diet. Korean J Physiol Pharmacol. 2010. 14:77–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Fenofibrate and Exercise on Metabolic Syndrome and Hepatic Steatosis in OLETF Rats
- Effects of Moderate Alcohol Intake in the Bladder of the Otsuka Long Evans Tokushima Fatty Diabetic Rats
- The Increase in Hepatic Uncoupling by Fenofibrate Contributes to a Decrease in Adipose Tissue in Obese Rats
- Femoral bone structure in Otsuka Long-Evans Tokushima Fatty rats
- The Effect of Leptin Level Fluctuations by a Repeated Fasting/Refeeding on the Leptin Sensitivity in OLETF Rats