J Korean Endocr Soc.
2008 Oct;23(5):310-318. 10.3803/jkes.2008.23.5.310.
The Effect of Leptin Level Fluctuations by a Repeated Fasting/Refeeding on the Leptin Sensitivity in OLETF Rats
- Affiliations
-
- 1Department of OBGY, College of Medicine, Yeungnam University, Korea.
- 2Department of Pediatrics, College of Medicine, Yeungnam University, Korea.
- 3Department of Physiology, College of Medicine, Yeungnam University, Korea.
- 4Department of Internal Medicine, College of Medicine, Yeungnam University, Korea.
- KMID: 1479223
- DOI: http://doi.org/10.3803/jkes.2008.23.5.310
Abstract
-
BACKGROUND: Leptin resistance is a common feature in obese subjects and animals, and this is commonly accompanied with hyperleptinemia. We speculated that one of the causes of leptin resistance is a persistently elevated leptin concentration and then we hypothesized that fluctuations of serum leptin would increase leptin sensitivity in the leptin-resistant state.
METHODS
We used a repeated fasting and refeeding (RFR) protocol to produce fluctuation in leptin levels in 7 month-old Otsuka Long-Evans Tokushima Fatty (OLETF) rats and Long-Evans Tokushima Otsuka (LETO) rats, We then measured the leptin sensitivity following an intracerebroventricular (i.c.v.) infusion of leptin.
RESULTS
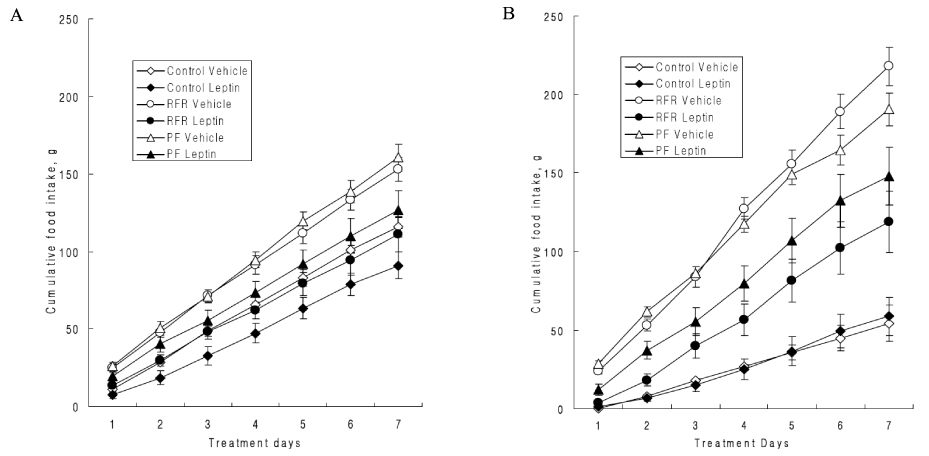
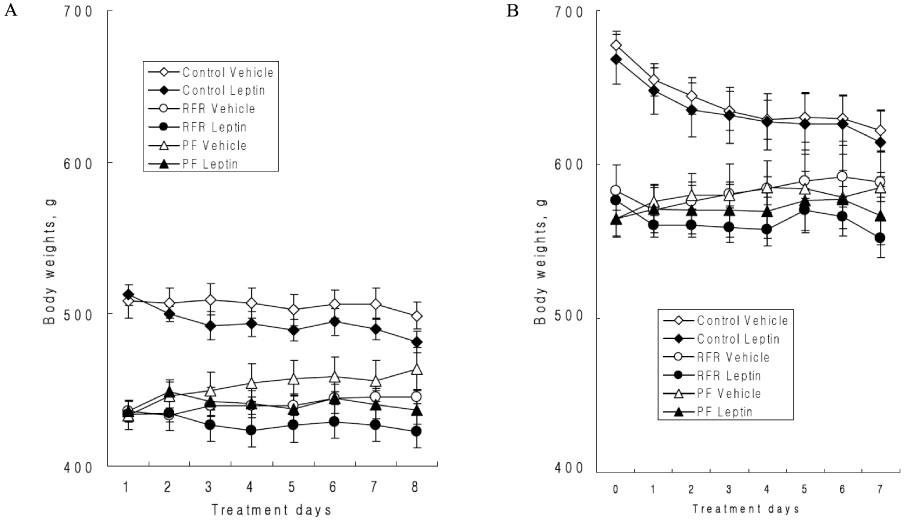
The OLETF rats exhibited severe visceral fat deposition, hyperleptinemia and leptin resistance. However, in the OLETF-RFR rats, the anorexic effect following i.c.v. leptin infusion was restored. Moreover, the visceral fat mass and serum leptin levels decreased, while the serum adiponectin levels were elevated in the OLETF-RFR rats compared to the OLETF-Control rats. The leptin receptor content in the hypothalamus increased in the OLETF-RFR rats compared to the OLETF-Control rats, and the leptin receptor content in the OLETF-RFR rats decreased compared to that in the the LETO-Control rats.
CONCLUSION
These results suggest that the intermittent suppression of the serum leptin level reversed the leptin resistance in OLEFT rats, and this may have occurred due to an increased number of leptin receptors in the hypothalamus.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Effect of Food Restriction on Appetite Regulating Hormones and Adiponectin Activity
Ki Hoon Kim, Hyun Kook Kim
Korean J Nutr. 2012;45(1):5-11. doi: 10.4163/kjn.2012.45.1.5.
Reference
-
1. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994. 372:425–432.2. Shek EW, Scarpace PJ. Resistance to the anorexic and thermogenic effects of centrally administrated leptin in obese aged rats. Regul Pept. 2000. 92:65–71.3. Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997. 99:385–390.4. Widdowson PS, Upton R, Buckingham R, Arch J, Williams G. Inhibition of food response to intracerebroventricular injection of leptin is attenuated in rats with diet-induced obesity. Diabetes. 1997. 46:1782–1785.5. Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decraesed cerebrospinalfluid/ serum leptin ratio in obesity, a possible mechanism for leptin resistance. Lancet. 1996. 348:159–161.6. Halaas JL, Booze C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci. 1997. 94:8878–8883.7. Scarpace PJ, Matheny M, Zhang Y, Shek EW, Prima V, Zolotukhin S, Tumer N. Leptin-induced leptin resistance reveals separate roles for the anorexic and thermogenic responses in weight maintenance. Endocrinology. 2002. 143:3026–3035.8. Fernández-Veledo S, Nieto-Vazquez I, de Castro J, Ramos MP, Brüderlein S, Möller P, Lorenzo M. Hyperinsulinemia induces insulin resistance on glucose and lipid metabolism in a human adipocytic cell line: paracrine interaction with myocytes. J Clin Endocrinol Metab. 2008. 93:2866–2876.9. Tachibana I, Akiyama T, Kanagawa K, Shiohara H, Furumi K, Watanabe N, Otsuki M. Defect in pancreatic exocrine and endocrine response to CCK in genetically diabetic OLETF rats. Am J Physiol. 1996. 270:G730–G737.10. Kim YW, Kim JY, Park YH, Park YH, Park SY, Won KC, Choi KH, Huh JY, Moon KH. Metformin restores leptin sensitivity in high fat fed obese rats with leptin resistance. Diabetes. 2006. 55:716–724.11. Yamashita S, Nakamura T, Shimomura I, Nishida M, Yoshida S, Kotani K, Kameda-Takemuara K, Tokunaga K, Matsuzawa Y. Insulin resistance and body fat distribution. Diabetes Care. 1996. 19:287–291.12. Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Choi DS, Baik SH, Choi KM. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem Biophys Res Commun. 2005. 336:747–753.13. Niimi M, Sato M, Yokote R, Tada S, Takahara J. Effects of central and peripheral injection of leptin on food intake and on brain Fos expression in the Otsuka Long-Evans Tokushima Fatty rat with hyperleptinaemia. J Neuroendocrinol. 1999. 11:605–611.14. Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001. 104:1111–1117.15. Kurose Y, Iqbal J, Rao A, Murata Y, Hasegawa Y, Terashima Y, Kojima M, Kangawa K, Clarke IJ. Changes in expression of the genes for the leptin receptor and the growth hormone-releasing peptide/ghrelin receptor in the hypothalamic arcuate nucleus with long-term manipulation of adiposity by dietary means. J Neuroendocrinol. 2005. 17:331–340.16. Keen-Rhinehart E, Kalra SP, Kalra PS. AAV-mediated leptin receptor installation improves energy balance and the reproductive status of obese female Koletsky rats. Peptides. 2005. 26:2567–2578.17. Wilsey J, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J Endocrinol. 2004. 181:297–306.18. Fernandez-Galaz C, Fernandez-Agullo T, Perez C, Peralta S, Arribas C, Andres A, Carrascosa JM, Ros M. Long-term food restriction prevents ageing-associated central leptin resistance in wistar rats. Diabetologia. 2002. 45:997–1003.19. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001. 7:941–946.20. Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004. 10:524–529.21. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999. 257:79–83.22. Prada PO, Hirabara SM, de Souza CT, Schenka AA, Zecchin HG, Vassallo J, Velloso LA, Carneiro E, Carvalheira JB, Curi R, Saad MJ. L-glutamine supplementation induces insulin resistance in adipose tissue and improves insulin signalling in liver and muscle of rats with diet-induced obesity. Diabetologia. 2007. 50:1949–1959.23. Lee CK, Kim YW. Changes of adipogenic and lipolytic activities following repeated fasting and refeeding in rat. Korean J Obesity. 2005. 14:16–21.24. Kmiec Z, Pokrywka L, Kotlarz G, Kubasik J, Szutowicz A, Mysliwski A. Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology. 2005. 51:357–362.25. Merl V, Peters A, Oltmanns KM, Kern W, Born J, Fehm HL, Schltes B. Serum adiponectin concentrations during a 72-hour fast in over- and normal-weight humans. Int J Obes Relat Metab Disord. 2005. 29:998–1001.26. Baratta R, Amato S, Degano C, Farina MG, Patane G, Vigneri R, Frittitta L. Adiponectin relationship with lipid metabolism is independent of body fat amss: Evidence from both cross-sectional and intervention studies. J Clin Endocrinol Metab. 2004. 89:2665–2671.27. Valsamakis G, McTernan PG, Chetty R, Daghri NA, Field A, Hanif W, Barnett AH, Kumar S. Modest weight loss and reduction in waist circumference after medical treatment are associated with favorable changes in serum adipokines. Metabolism. 2004. 53:430–434.28. Delporte ML, Mkadem SAE, Quisquarter M, Brichard SM. Leptin treatment markedly increased plasma adiponectin but barely decreased plasma resistin of ob/ob mice. Am J Physiol Endocrinol Metab. 2004. 287:E446–E453.29. Kim TS, Freake HC. High carbohydrate diet and starvation regulate lipogenic mRNA in rats in a tissue-specific manner. J Nutr. 1996. 126:611–617.30. Kochan Z, Karbowska J, Swierczynski J. Unusual increase of lipogenesis in rat white adipose tissue after multiple cycles of starvation-refeeding. Metabolism. 1997. 46:10–17.31. Kim YW, Scarpace PJ. Repeated fasting/refeeding elevates plasma leptin without increasing fat mass in rats. Physiol Behav. 2003. 78:459–464.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Leptin Level Fluctuations by a Repeated Fasting/Refeeding on the Leptin Sensitivity in OLETF Rats
- Changes of Lipogenic and Lipolytic Activities Following Repeated Fasting and Refeeding in Rat
- The Effect of Food Restriction on Appetite Regulating Hormones and Adiponectin Activity
- Metformin Enhances Leptin Sensitivity in Aged Rats
- Naloxone Increases the Anorexic Effect of MTII in OLETF Rats