J Korean Endocr Soc.
2007 Jun;22(3):192-202. 10.3803/jkes.2007.22.3.192.
The Effect of Fenofibrate and Exercise on Metabolic Syndrome and Hepatic Steatosis in OLETF Rats
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine,Dong-A University, Korea.
- 2Department of Pharmacology, College of Medicine,Dong-A University, Korea.
- 3Department of Pathology, College of Medicine,Dong-A University, Korea.
- 4Medical Sciences Research Institutes, College of Medicine,Dong-A University, Korea.
- 5Department of Internal Medicine, Baptist Hospital, Korea.
- 6Department of Internal Medicine, Suyeong Hanseo Hospital, Korea.
- 7Center for Human Nutrition, University of Texas Southwestern Medical Center, USA.
- KMID: 1512031
- DOI: http://doi.org/10.3803/jkes.2007.22.3.192
Abstract
-
BACKGROUND: The aim of this study is to verify the effects of fenofibrate monotherapy and fenofibrate combined with exercise for improving metabolic syndrome and hepatic steatosis.
METHODS
Thirty-four weeks old OLETF rats (Otsuka Long-Evans Tokushima Fatty Rats, n = 20) were divided four groups: the regular diet group (n = 5, DD group), the exercise group (n = 5, DE group), the fenofibrate (100 mg/kg) treated group (n = 5, DF group) and the combination treatment group {fenofibrate and exercise (n = 5, EF group)}. After 5 weeks of treatment, blood was drawn for measuring the blood glucose, insulin, lipid and leptin levels. All the subjects were sacrificed for assessment of their body adiposity and hepatic steatosis.
RESULTS
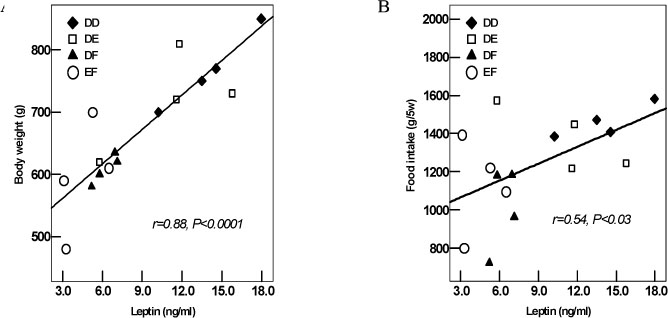
The total amount of food intake, body weight and total body weight of the rat were significantly decreased in the EF and DF groups compared to the DD group. The plasma triglyceride and glucose levels were significantly decreased in the EF and DF groups compared to the DD group. The HOMA-IR of EF, DF and DE groups were significantly decreased compared with that of the DD group. The plasma leptin levels of the EF and DF groups were significantly decreased compared with those of the DD and DE groups. The hepatic steatosis index was significantly decreased in the EF and DF groups compared to the DD and DE groups.
CONCLUSION
Fenofibrate monotherapy was effective to control three major components (obesity, hypertriglyceridemia and hyperglycemia) of metabolic syndrome and hepatic steatosis in OLETF rats. Exercise combined with fenofibrate treatment showed an additional effect compared to that of fenofibrate monotherapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Wilson PW, Grundy SM. The metabolic syndrome: practical guide to origins and treatment: Part I. Circulation. 2003. 108:1422–1424.2. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999. 116:1413–1419.3. Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000. 132:112–117.4. Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004. 2:262–265.5. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001. 50:1844–1850.6. Henriksen EJ. Invited review: Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. 2002. 93:788–796.7. Ivy JL. Muscle insulin resistance amended with exercise training: role of GLUT4 expression. Med Sci Sports Exerc. 2004. 36:1207–1211.8. Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003. 52:2191–2197.9. Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc. 2001. 33:6 Suppl. S521–S527.10. Couillard C, Despres JP, Lamarche B, Bergeron J, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arterioscler Thromb Vasc Biol. 2001. 21:1226–1232.11. Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003. 38:1008–1017.12. Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, Liang TJ, Yanovski JA, Kleiner DE, Hoofnagle JH. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004. 39:188–196.13. Ginsberg HN. Treatment for patients with the metabolic syndrome. Am J Cardiol. 2003. 91(7A):29E–39E.14. Grundy SM, Hansen B, Smith SC Jr, Cleeman JI, Kahn RA. American Heart Association. National Heart, Lung, and Blood Institute. American Diabetes Association. Clinical management of metabolic syndrome: report of the American HeartAssociation/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol. 2004. 24:e19–e24.15. Lee HJ, Choi SS, Park MK, An YJ, Seo SY, Kim MC, Hong SH, Hwang TH, Kang DY, Garber AJ, Kim DK. Fenofibrate lowers abdominal and skeletal adiposity and improves insulin sensitivity in OLETF rats. Biochem Biophys Res Commun. 2002. 296:293–299.16. Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998. 98:2088–2093.17. Tsutsumi And M, Takase S. Effect of fenofibrate on fatty liver in rats treated with alcohol. Alcohol Clin Exp Res. 2001. 25:6 Suppl. 75S–79S.18. Hamada N, Ogawa Y, Saibara T, Murata Y, Kariya S, Nishioka A, Terashima M, Inomata T, Yoshida S. Toremifene-induced fatty liver and NASH in breast cancer patients with breast-conservation treatment. Int J Oncol. 2000. 17:1119–1123.19. Saengsirisuwan V, Kinnick TR, Schmit MB, Henriksen EJ. Interactions of exercise training and lipoic acid on skeletal muscle glucose transport in obese Zucker rats. J Appl Physiol. 2001. 91:145–153.20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and b cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.21. Man ZW, Zhu M, Noma Y, Toide K, Sato T, Asahi Y, Hirashima T, Mori S, Kawano K, Mizuno A, Sano T, Shima K. Impaired beta-cell function and deposition of fat droplets in the pancreas as a consequence of hypertriglyceridemia in OLETF rat, a model of spontaneous NIDDM. Diabetes. 1997. 46:1718–1724.22. Yagi K, Kim S, Wanibuchi H, Yamashita T, Yamamura Y, Iwao H. Characteristics of diabetes, blood pressure, and cardiac and renal complications in Otsuka Long-Evans Tokushima Fatty rats. Hypertension. 1997. 29:728–735.23. Nakaya Y, Minami A, Harada N, Sakamoto S, Niwa Y, Ohnaka M. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am J Clin Nutr. 2000. 71:54–58.24. Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, Derudas B, Herbert JM, Winegar DA, Willson TM, Fruchart JC, Berge RK, Staels B. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000. 275:16638–16642.25. Meertens LM, Miyata KS, Cechetto JD, Rachubinski RA, Capone JP. A mitochondrial ketogenic enzyme regulates its gene expression by association with the nuclear hormone receptor PPARalpha. EMBO J. 1998. 17:6972–6978.26. Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol. 1988. 255(6 Pt 2):R974–R981.27. Yamamoto K, Fukuda N, Zhang L, Sakai T. Altered hepatic metabolism of fatty acids in rats fed a hypolipidaemic drug, fenofibrate. Pharmacol Res. 1996. 33:337–342.28. Larsen PJ, Jensen PB, Sorensen RV, Larsen LK, Vrang N, Wulff EM, Wassermann K. Differential influences of peroxisome proliferator activated receptors γ and -α on food intake and energy homeostasis. Diabetes. 2003. 52:2249–2259.29. Silver AJ, Flood JF, Song AM, Morley JE. Evidence for a physiological role for CCK in the regulation of food intake in mice. Am J Physiol. 1989. 256(3 Pt 2):R646–R652.30. Tomita H, Miyasaka K, Jimi A, Mishima Y, Funakoshi A. Lack of effect of cholecystokinin receptor antagonist (CR1505) on recovery of experimental pancreatitis after pancreatic duct occlusion in rats. Pancreas. 1994. 9:638–645.31. Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996. 37:907–925.32. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004. 114:147–152.33. Medina J, Fernandez-Salazar LI, Garcia-Buey L, Moreno-Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004. 27:2057–2066.34. Chen MT, Kaufman LN, Spennetta T, Shrago E. High fat-feeding to rats on the interrelationship of body weight, plasma insulin, and fatty acyl-coenzyme A esters in liver and skeletal muscle. Metabolism. 1992. 41:564–569.35. Lam TK, Yoshii H, Haber CA, Bogdanovic E, Lam L, Fantus IG, Giacca A. Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-δ. Am J Physiol Endocrinol Metab. 2002. 283:E682–E691.36. Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver isassociated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002. 87:3023–3028.37. Ryysy L, Hakkinen AM, Goto T, Vehkavaara S, Westerbacka J, Halavaara J, Yki-Jarvinen H. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000. 49:749–758.38. Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, Samarasinghe D, George J. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002. 36:403–409.39. An YJ, Lee HJ, Park MK, Lee KI, Koh IY, Jung DS, Kang AY, Kim DK. Exercise and fenofibrate reduces body adiposity synergistically in OLETF rats. J Kor Diabetes Assoc. 2004. 28:131–138.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Fenofibrate and Exercise on Metabolic Syndrome and Hepatic Steatosis
- Exercise and Fenofibrate Reduces Body Adiposity Synergistically in OLETF Rats
- Prevention of Diabetes by Fenofibrate in OLETF Rats: Hepatic Mechanism for Reducing Visceral Adiposity
- Hepatic Fibrosis and Steatosis in Metabolic Syndrome
- Reduction of Food Intake by Fenofibrate is Associated with Cholecystokinin Release in Long-Evans Tokushima Rats