J Korean Med Sci.
2015 Sep;30(9):1313-1320. 10.3346/jkms.2015.30.9.1313.
Effects of Moderate Alcohol Intake in the Bladder of the Otsuka Long Evans Tokushima Fatty Diabetic Rats
- Affiliations
-
- 1Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. uroljy@catholic.ac.kr
- 2Department of Psychiatry, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Catholic Prostate Institute, The Catholic University of Korea, Seoul, Korea.
- 4Department of Bioinformatics, The Catholic University of Korea, Seoul, Korea.
- KMID: 2344158
- DOI: http://doi.org/10.3346/jkms.2015.30.9.1313
Abstract
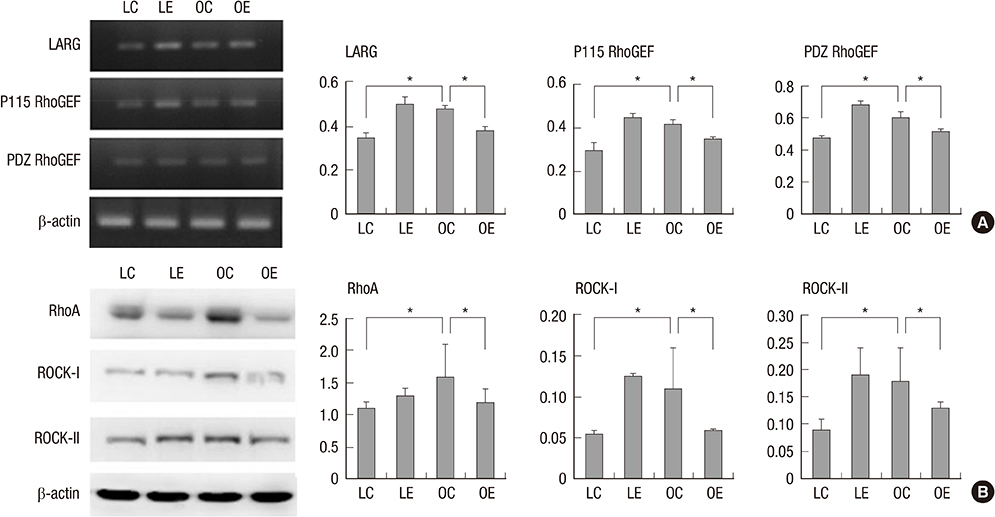
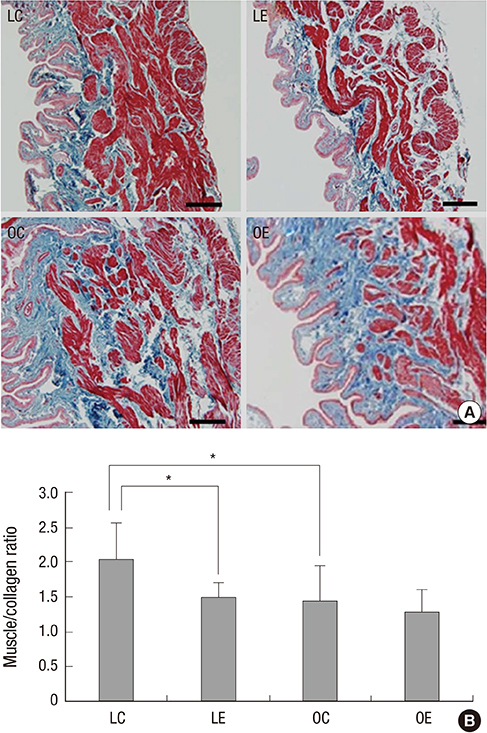
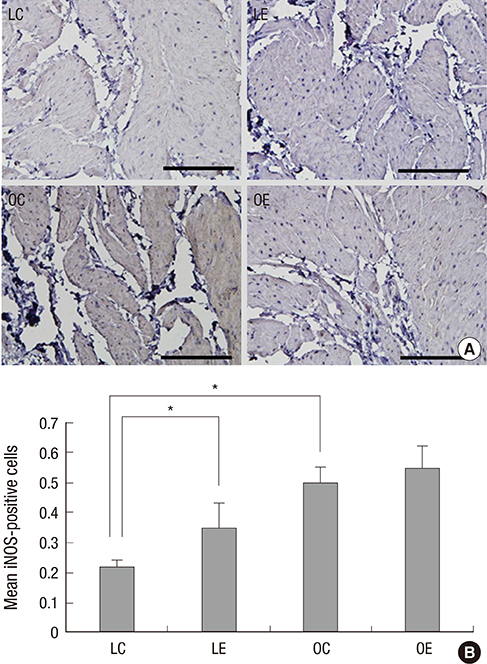
- Diabetes is related with a number of cystopathic complications. However, there have been no studies about the influence of alcohol consumption in the bladder of type 2 diabetes. Thus, we investigated the effect of moderate alcohol intake in the bladder of the Otsuka Long Evans Tokushima Fatty (OLETF) diabetic rat. The non-diabetic Long-Evans Tokushima Otsuka (LETO, n=14) and the OLETF control group (n=14) were fed an isocaloric diet; the LETO (n=14) and the OLETF ethanol group (n=14) were fed 36% ethanol 7 g/kg/day. After ten weeks, muscarinic receptors, RhoGEFs, myogenic change, and the level of oxidative stress were evaluated. Moderate alcohol intake significantly decreased excessive muscarinic receptor and Rho kinase expressions in the OLETF rats compared with the LETO rats. In addition, iNOS and collagen expression were not changed in the OLETF rats in spite of alcohol consumption. Superoxide dismutase levels, which is involved in antioxidant defense, in the LETO rats were significantly decreased after alcohol consumption, however those in the OLETF rats were similar. Moderate alcohol consumption reduces the oxidative stress, and may prevent molecular and pathologic changes of the bladder of rats with type 2 diabetes.
Keyword
MeSH Terms
Figure
Reference
-
1. Frimodt-Møller C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med. 1980; 92:318–321.2. Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005; 95:733–738.3. Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci U S A. 2000; 97:9579–9584.4. Igawa Y, Zhang X, Nishizawa O, Umeda M, Iwata A, Taketo MM, Manabe T, Matsui M, Andersson KE. Cystometric findings in mice lacking muscarinic M2 or M3 receptors. J Urol. 2004; 172:2460–2464.5. Tammela TL, Leggett RE, Levin RM, Longhurst PA. Temporal changes in micturition and bladder contractility after sucrose diuresis and streptozotocin-induced diabetes mellitus in rats. J Urol. 1995; 153:2014–2021.6. Cheng JT, Yu BC, Tong YC. Changes of M3-muscarinic receptor protein and mRNA expressions in the bladder urothelium and muscle layer of streptozotocin-induced diabetic rats. Neurosci Lett. 2007; 423:1–5.7. Nobe K, Yamazaki T, Tsumita N, Hashimoto T, Honda K. Glucose-dependent enhancement of diabetic bladder contraction is associated with a rho kinase-regulated protein kinase C pathway. J Pharmacol Exp Ther. 2009; 328:940–950.8. Yokoi K, Ohmura M, Kondo A, Miyake K, Saito M. Effects of ethanol on in vivo cystometry and in vitro whole bladder contractility in the rat. J Urol. 1996; 156:1489–1491.9. Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med. 2004; 140:211–219.10. Ting JW, Lautt WW. The effect of acute, chronic, and prenatal ethanol exposure on insulin sensitivity. Pharmacol Ther. 2006; 111:346–373.11. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–553.12. Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005; 28:719–725.13. Kleinhenz DJ, Sutliff RL, Polikandriotis JA, Walp ER, Dikalov SI, Guidot DM, Hart CM. Chronic ethanol ingestion increases aortic endothelial nitric oxide synthase expression and nitric oxide production in the rat. Alcohol Clin Exp Res. 2008; 32:148–154.14. Sebastian BM, Nagy LE. Decreased insulin-dependent glucose transport by chronic ethanol feeding is associated with dysregulation of the Cbl/TC10 pathway in rat adipocytes. Am J Physiol Endocrinol Metab. 2005; 289:E1077–E1084.15. Tong YC, Cheng JT, Hsu CT. Alterations of M(2)-muscarinic receptor protein and mRNA expression in the urothelium and muscle layer of the streptozotocin-induced diabetic rat urinary bladder. Neurosci Lett. 2006; 406:216–221.16. Teixeira CE, Jin L, Ying Z, Palmer T, Webb RC. Ca2+ sensitization and the regulation of contractility in rat anococcygeus and retractor penis muscle. Biochem Pharmacol. 2005; 69:1483–1492.17. Bae WJ, Ha US, Kim KS, Kim SJ, Cho HJ, Hong SH, Lee JY, Wang Z, Hwang SY, Kim SW. Effects of KH-204 on the expression of heat shock protein 70 and germ cell apoptosis in infertility rat models. BMC Complement Altern Med. 2014; 14:367.18. Longhurst PA, Belis JA. Abnormalities of rat bladder contractility in streptozotocin-induced diabetes mellitus. J Pharmacol Exp Ther. 1986; 238:773–777.19. Luheshi GN, Zar MA. The effect of streptozotocin-induced diabetes on cholinergic motor transmission in the rat urinary bladder. Br J Pharmacol. 1991; 103:1657–1662.20. Tong YC, Hung YC, Lin SN, Cheng JT. Alterations in urinary bladder synaptosomal neurotransmitter concentrations in two-week streptozotocin-induced diabetic rats. Neurosci Lett. 1996; 206:165–168.21. Latifpour J, Gousse A, Kondo S, Morita T, Weiss RM. Effects of experimental diabetes on biochemical and functional characteristics of bladder muscarinic receptors. J Pharmacol Exp Ther. 1989; 248:81–88.22. Miyamae K, Yoshida M, Inadome A, Murakami S, Otani M, Iwashita H, Masunaga K, Ueda S. Acetylcholine release from urinary bladder smooth muscles of non-insulin-dependent diabetic rats. Urol Int. 2004; 73:74–80.23. Yono M, Latifpour J, Yoshida M, Ueda S. Age-related alterations in the biochemical and functional properties of the bladder in type 2 diabetic GK rats. J Recept Signal Transduct Res. 2005; 25:147–157.24. Rösen P, Du X, Tschöpe D. Role of oxygen derived radicals for vascular dysfunction in the diabetic heart: prevention by alpha-tocopherol? Mol Cell Biochem. 1998; 188:103–111.25. Szaleczky E, Prechl J, Fehér J, Somogyi A. Alterations in enzymatic antioxidant defence in diabetes mellitus--a rational approach. Postgrad Med J. 1999; 75:13–17.26. Kanika ND, Chang J, Tong Y, Tiplitsky S, Lin J, Yohannes E, Tar M, Chance M, Christ GJ, Melman A, et al. Oxidative stress status accompanying diabetic bladder cystopathy results in the activation of protein degradation pathways. BJU Int. 2011; 107:1676–1684.27. Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000; 26:163–176.28. Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990; 173:932–939.29. Wong A, Dukic-Stefanovic S, Gasic-Milenkovic J, Schinzel R, Wiesinger H, Riederer P, Münch G. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur J Neurosci. 2001; 14:1961–1967.30. Rojas A, Caveda L, Romay C, López E, Valdés S, Padrón J, Glaría L, Martínez O, Delgado R. Effect of advanced glycosylation end products on the induction of nitric oxide synthase in murine macrophages. Biochem Biophys Res Commun. 1996; 225:358–362.31. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301:2129–2140.32. Friedman LA, Kimball AW. Coronary heart disease mortality and alcohol consumption in Framingham. Am J Epidemiol. 1986; 124:481–489.33. Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJ, Grobbee DE, Kluft C, Hendriks HF. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, and insulin sensitivity. Diabetes Care. 2004; 27:184–189.34. Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004; 89:2563–2568.35. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005; 26:439–451.36. Ding M, Carrão AC, Wagner RJ, Xie Y, Jin Y, Rzucidlo EM, Yu J, Li W, Tellides G, Hwa J, et al. Vascular smooth muscle cell-derived adiponectin: a paracrine regulator of contractile phenotype. J Mol Cell Cardiol. 2012; 52:474–484.37. Nobe K, Fujii A, Saito K, Negoro T, Ogawa Y, Nakano Y, Hashimoto T, Honda K. Adiponectin enhances calcium dependency of mouse bladder contraction mediated by protein kinase Calpha expression. J Pharmacol Exp Ther. 2013; 345:62–68.38. Daneshgari F, Liu G, Imrey PB. Time dependent changes in diabetic cystopathy in rats include compensated and decompensated bladder function. J Urol. 2006; 176:380–386.39. Lee JH, Suh HJ, Cho HJ, Lee YS, Kim HW, Kim SH, Kim SW, Hwang TK, Lee SJ, Lee JY. Functional, morphologic, and molecular biological changes in the bladder of OLETF diabetic rats according to duration of diabetes mellitus. Korean J Urol. 2009; 50:387–395.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Caloric Restriction on the Expression of PGC-1 and PPARs mRNA in Liver of Otsuka Long-Evans Tokushima Fatty Rats
- Ultrastructural Changes in Corneas of Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats
- Femoral bone structure in Otsuka Long-Evans Tokushima Fatty rats
- Beneficial Effects of Thiazolidinediones on Diabetic Nephropathy in OLETF Rats
- Response: Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats (Korean Diabetes J 2010;34:191-9)