J Korean Med Sci.
2005 Aug;20(4):663-669. 10.3346/jkms.2005.20.4.663.
The Role of Inducible Nitric Oxide Synthase Following Spinal Cord Injury in Rat
- Affiliations
-
- 1Department of Oral Pathology, School of Medicine, Kyungpook National University, Daegu, Korea.
- 2Department of Oral Microbiology, School of Dentistry, School of Medicine, Kyungpook National University, Daegu, Korea.
- 3Department of Pathology, School of Medicine, Kyungpook National University, Daegu, Korea. yksohn@knu.ac.kr
- 4Department of Pathology, School of Medicine, Samsung Cheil Hospital, Sunkyunkwan University, School of Medicine, Seoul, Korea.
- KMID: 1712751
- DOI: http://doi.org/10.3346/jkms.2005.20.4.663
Abstract
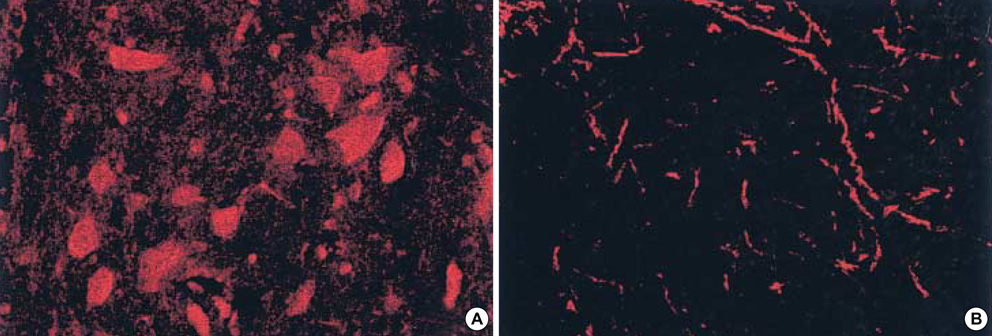
- Acute spinal cord injury (SCI) is two-step process that first involves the primary mechanical injury and then the secondary injury is induced by various biochemical reactions. Apoptosis is one of secondary SCI mechanisms and it is thought to play an important role for the delayed neuronal injury. The enhanced formation of nitric oxide (NO) via inducible nitric oxide synthase (iNOS) has been implicated in the pathogenesis of apoptosis in SCI. The level of .iNOS mRNA peaked at 6 hr after SCI and it declined until 72 hr after SCI in a rat model. Double-immunofluorescence staining revealed that iNOS positive cells were stained for ED-1, synaptophysin, GFAP, and oligodendrocyte marker. The terminal deoxynucleotidyl-transferase-mediated dUDP-biotin nick end-labeling (TUNEL) positive cell count was higher for the 72 hr post-SCI group than for the 24 hr post-SCI group. This cell count was also higher going in the caudal direction than in the rostral direction from the epicenter, and especially for the 72 hr group. Treatment with a selective iNOS inhibitor resulted in the reduction of TUNEL-positive cells at the lesion site. These findings suggest that nitric oxide generated by the iNOS of macrophages, neurons, oligodentrocytes, and astrocytes plays an important role for the acute secondary SCI that results from apoptotic cell death.
MeSH Terms
-
Analysis of Variance
Animals
Apoptosis
Comparative Study
Glial Fibrillary Acidic Protein/analysis
In Situ Nick-End Labeling
Microscopy, Fluorescence
RNA, Messenger/genetics/metabolism
Rats
Rats, Sprague-Dawley
Research Support, Non-U.S. Gov't
Reverse Transcriptase Polymerase Chain Reaction
Spinal Cord/chemistry/enzymology/pathology
Spinal Cord Injuries/*enzymology/pathology/physiopathology
Time Factors
Figure
Cited by 1 articles
-
Preconditioning of isoflurane on spinal cord ischemia can increase the number of inducible nitric oxide synthase-expressing motor neurons in rat
Yun-Hee Sung, Sang-Hak Lee, Joon-Kyung Sung, Jin-Hee Han, Hong Kim, Chang-Ju Kim, Jong-Man Kang
Korean J Anesthesiol. 2010;58(1):70-75. doi: 10.4097/kjae.2010.58.1.70.
Reference
-
1. Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, Part I: Pathophysiologic mechanisms. Clin Neurophamacol. 2001. 24:254–264.
Article2. Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995. 5:407–413.
Article3. Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991. 75:15–26.
Article4. Demopoulos HB, Flamm ES, Seligman ML, Mitamura JA, Ransohoff J. Popp AJ, editor. Membrane perturbations in central nervous system injury: Theoretical basis for free radical damage and a review of the experimental data. Neural Trauma. 1979. New York: Raven Press;63–78.5. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001. 26:24 Suppl. S2–S12.
Article6. Freeman RS, Estus S, Johnson EM Jr. Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron. 1994. 12:343–355.
Article7. Rotello RJ, Fernandez PA, Yuan J. Anti-apogens and anti-engulfens: monoclonal antibodies reveal specific antigens on apoptotic and engulfment cells during chicken embryonic development. Development. 1994. 120:1421–1431.
Article8. Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997. 3:73–76.
Article9. Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998. 89:911–920.
Article10. Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997. 50:798–808.
Article11. Matsuyama Y, Sato K, Kamiya M, Yano J, Iwata H, Isobe K. Nitric oxide: a possible etiologic factor in spinal cord cavitation. J Spinal Disord. 1998. 11:248–252.12. Dawson VL, Kizushi VM, Huang PL, Snyder SH, Dawson TM. Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci. 1996. 16:2479–2487.
Article13. Hamada Y, Ikata T, Katoh S, Tsuchiya K, Niwa M, Tsutsumishita Y, Fukuzawa K. Roles of nitric oxide in compression injury of rat spinal cord. Free Radic Biol Med. 1996. 20:1–9.14. Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997. 28:1283–1288.
Article15. Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitrosocompounds. Nature. 1993. 364:626–632.
Article16. Allen A. Surgery of experimental lesions of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. JAMA. 1911. 57:878–880.17. Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res. 2000. 85:114–122.
Article18. Wada K, Chatzipanteli K, Kraydieh S, Busto R, Dietrich WD. Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats. Neurosurgery. 1998. 43:1427–1436.
Article19. Wada K, Chatzipanteli K, Busto R, Dietrich WD. Effects of L-NAME and 7-NI on NOS catalytic activity and behavioral outcome after traumatic brain in the rat. J Neurotrauma. 1999. 16:203–212.20. Wolman L. The disturbances of circulation in traumatic paraplegia in acute and late stages: a pathological study. Paraplegia. 1965. 59:213–226.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Involvement of Fibronectin in the Migration of Macrophage and Expression of Nitric Oxide Synthase in the BCG induced Inflammatory Sites in Rat Bladder
- The Effect of Nitric Oxide on Mechanical and Theraml Allodynia in Neuropathic Pain Model of Rat
- Alteration of Nitric Oxide Synthase Subtype Expression in Contralateral Testis of Rat in Response to Unilateral Testicular Torsion Followed by Detorsion
- Expression of Constitutive Nitric Oxide Synthase by Gastrointestinal Epithelial Cells
- The Potential Role of Nitric Oxide in Halting Cancer Progression Through Chemoprevention