J Korean Med Sci.
2005 Aug;20(4):548-554. 10.3346/jkms.2005.20.4.548.
Epigallocatechin-3-gallate Suppresses Galactose-alpha1,4-galactose-beta1,4-glucose Ceramide Expression in TNF-alpha Stimulated Human Intestinal Epithelial Cells Through Inhibition of MAPKs and NF-kappa B
- Affiliations
-
- 1Department of Microbiology and Immunology, and Medical Research Institute, and Laboratory of Dendritic Differentiation and Regulation, Pusan National University College of Medicine, Busan, Korea. immunpym@pusan.ac.kr
- 2School of Applied Marine Science, College of Ocean Science, Cheju National University, Jeju, Korea.
- 3Department of Microbiology, College of Natural Science, Pusan National University, Busan, Korea.
- KMID: 1712731
- DOI: http://doi.org/10.3346/jkms.2005.20.4.548
Abstract
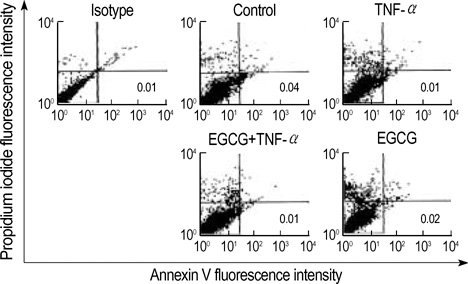
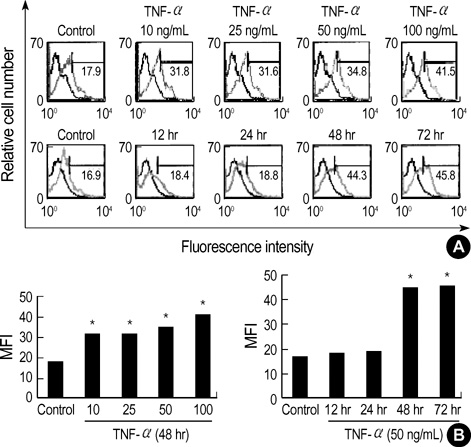
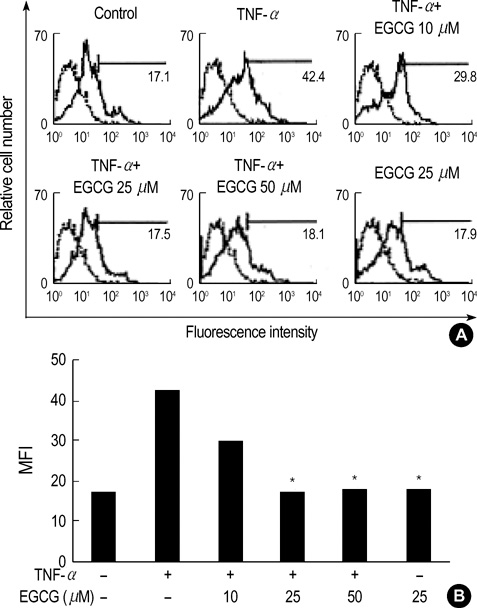
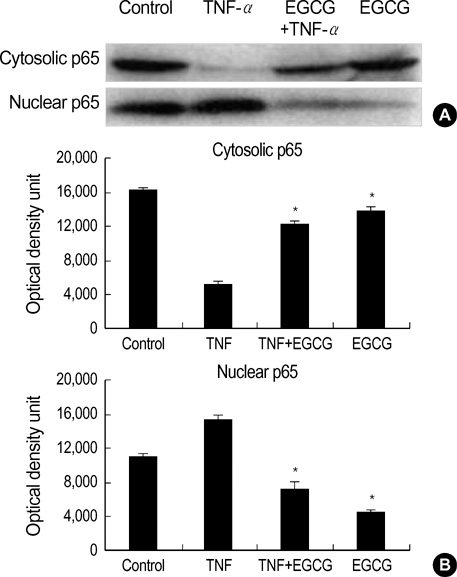
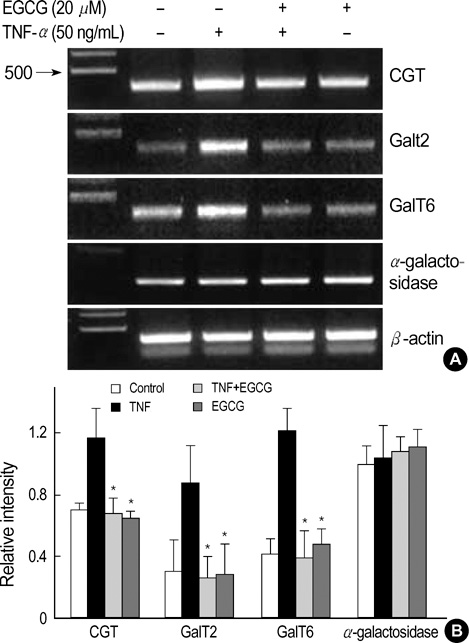
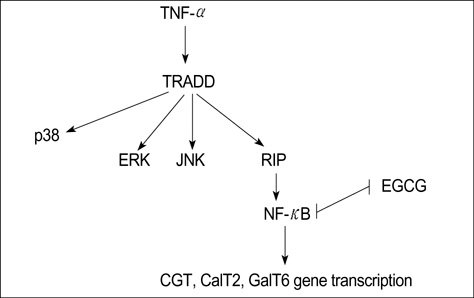
- Intestinal epithelial cells (IECs) have been known to produce galactose-alpha1,4-galactose-beta1,4-glucose ceramide (Gb3) that play an important role in the mucosal immune response. The regulation of Gb3 is important to prevent tissue damage causing shiga like toxin. Epigallocatechin-3-gallate (EGCG) has been studied as anti-carcinogenic, anti-oxidant, anti-angiogenic, and anti-viral activities, and anti-diabetic. However, little is known between the expressions of Gb3 on IECs. The aim of this study was to examine the inhibitory effect of EGCG, a major ingredient of green tea, on Gb3 production via mitogen-activated protein kinases (MAPKs) and nuclear factor-kappa B (NF-kappa B) in the TNF-alpha stimulated human colon epithelial cells, HT29. To investigate how Gb3 is regulated, ceramide glucosyltransferase (CGT), lactosylceramide synthase (GalT2), and Gb3 synthase (GalT6) were analyzed by RT-PCR in HT 29 cells exposed to TNF-alpha in the presence or absence of EGCG. EGCG dose-dependently manner, inhibits TNF-alpha induced Gb3 expression by blocking in both the MAPKs and NF-kappaB pathways in HT29 cells. TNF-alpha enhanced CGT, GalT2 and GalT6 mRNA levels and EGCG suppressed the level of these enzymes enhanced by TNF-alpha treatment.
Keyword
MeSH Terms
-
Apoptosis/drug effects
Blotting, Western
Catechin/*analogs & derivatives/pharmacology
Cell Nucleus/drug effects/metabolism
Cell Proliferation/drug effects
Dose-Response Relationship, Drug
Epithelial Cells/drug effects/metabolism/pathology
Flow Cytometry
Galactosyltransferases/genetics
Gene Expression Regulation, Enzymologic/drug effects
Glucosyltransferases/genetics
HT29 Cells
Humans
Intestinal Mucosa/drug effects/metabolism/pathology
Mitogen-Activated Protein Kinases/antagonists & inhibitors/*metabolism
NF-kappa B/antagonists & inhibitors/*metabolism
Phosphorylation/drug effects
Protein Transport/drug effects
RNA, Messenger/genetics/metabolism
Research Support, Non-U.S. Gov't
Reverse Transcriptase Polymerase Chain Reaction
Trihexosylceramides/*biosynthesis
Tumor Necrosis Factor-alpha/*pharmacology
Figure
Reference
-
1. Paton JC, Paton AW. Pathogensis and diagnosis of shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998. 11:450–479.2. Robson WL, Leung AK, Kaplan BS. Hemolytic-uremic syndrome. Curr Probl Pediatr. 1993. 23:16–33.
Article3. Lingwood CA. Verotoxin-binding in human renal sections. Nephron. 1994. 66:21–28.
Article4. Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988. 171:45–50.
Article5. Jacewicz M, Clausen H, Nudelman E, Donohue-Rolfe A, Keusch GT. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J Exp Med. 1986. 163:1391–1404.
Article6. Lindberg AA, Brown JE, Stromberg N, Westling-Ryd M, Schultz JE, Karlsson KA. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987. 262:1779–1785.
Article7. Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral Res. 1993. 21:289–299.
Article8. Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993. 85:1038–1049.
Article9. Katiyar SK, Mukhtar H. Tea antioxidants in cancer chemoprevention. J Cell Biochem Suppl. 1997. 27:59–67.
Article10. Sakanaka M, Kim M, Taniguchi M, Yamanoto T. Anti-bacteria substances in Japanese green tea extract against Streptococcus mutants, a carcinogenic bacterium. Agric Biol Chem. 1989. 53:2307–2311.11. Toda M, Okubo S, Ikigai H, Suzuki T, Suzuki Y, Shimamura T. The protective activity of tea against infection by Vibrio cholerae O1. J Appl Bacteriol. 1991. 70:109–112.12. Toda M, Okubo S, Hiyoshi R, Shimamura T. The bactericidal activity of tea and coffee. Lett Appl Microbiol. 1989. 8:123–125.
Article13. Ahn SC, Kim GY, Kim JH, Baik SW, Han MK, Lee HJ, Moon DO, Lee CM, Kang JH, Kim BH, Oh YH, Park YM. Epigallocatechin-3-gallate, constituent of green tea, suppresses the LPS-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinase and NF-κB. Biochem Biophys Res Commun. 2004. 313:148–155.14. Kundu JK, Na HK, Chun KS, Kim YK, Lee SJ, Lee SS, Lee OS, Sim YC, Surh YJ. Inhibition of phorbol ester-induced COX-2 expression by epigallocatechin gallate in mouse skin and cultured human mammary epithelial cells. J Nutr. 2003. 133:3805S–3810S.
Article15. Ahn Y, Hadizadeh KR, Seul C, Yun YP. Epigallocathechin-3-gallate selectively inhibits the PDGF-BB-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of cis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A127). Mol Biol Cell. 1999. 10:1093–1104.16. Jacewicz MS, Acheson DW, Mobassaleh M, Donohue-Rolfe A, Balasubramanian KA, Keusch GT. Maturational regulation of globotriaosylceramide, the Shiga-like toxin 1 receptor, in cultured human gut epithelial cells. J Clin Invest. 1995. 96:1328–1335.
Article17. Proulx F, Seidman EG, Karpman D. Pathogenesis of shiga toxin-associated hemolytic uremic syndrome. Pediatr Res. 2001. 50:163–171.
Article18. Acheson DW, Moore R, De Breucker S, Lincicome L, Jacewicz M, Skutelsky E, Keusch GT. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect Immun. 1996. 64:3294–3300.
Article19. Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993. 61:4569–4574.
Article20. Klimpel GR, Asuncion M, Haithcoat J, Niesel DW. Cholera toxin and Salmonella typhimurium induce different cytokine profiles in the gastrointestinal tract. Infect Immun. 1995. 63:1134–1137.
Article21. Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995. 63:289–296.
Article22. McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993. 123:895–907.
Article23. Stricklett PK, Hughes AK, Ergonul Z, Kohan DE. Molecular basis for up-regulation by inflammatory cytokines of shiga toxin1 and globotriaosylceramide expression. J Infect Dis. 2002. 186:976–982.24. Kim JA, Kim DK, Jin Tae, Kang OH, Choi YA, Choi SC, Kim TH, Nah YH, Choi SJ, Kim YH, Bae KH, Lee YM. Acanthoic acid inhibits IL-8 production via MAPKs and NF-kappaB in TNF-alpha-stimulated human intestinal epithelial cell line. Clin Chim Acta. 2004. 342:193–202.25. Tesh VL. Virulence of enterohemorrhagic Escherichia coli: role of molecular crosstalk. Trends Microbiol. 1998. 6:228–233.
Article26. Van de Kar NC, Monnens LA, Van Hinsbergh VW. Tumor necrosis factor and interleukin 1 induce expression of the glycolipid verotoxin receptor in human endothelial cells. Implications for the pathogenesis of the haemolytic uraemic syndrome. Behring Inst Mitt. 1993. 92:202–209.27. Trompezinski S, Denis A, Schmitt D, Viac J. Comparative effects of polyphenols from green tea (EGCG) and soybean (genistein) on VEGF and IL-8 release from normal human keratinocytes stimulated with the proinflammatory cytokine TNF-alpha. Arch Dermatol Res. 2003. 295:112–126.28. Christine A, Wanke MD. Recombinant human tumor necrosis factor and recombinant murine intherleukin-1 alter the binking of Escherichia coli to intestine, mucin glycoprotein, and the HT29-C1 intestinal cell line. Nutrition. 1997. 13:959–964.29. Chen W, Dong Z, Valcic S, Timmermann BN, Bowden GT. Inhibition of ultraviolet B-induced c-fos gene expression and p38 mitogen-activated protein kinase activation by (-)-epigallocatechin gallate in human keratinocyte cell line. Mol Carcinog. 1999. 24:79–84.30. Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000. 23:605–612.31. Singh R, Ahmed S, Malemud CJ, Goldberg VM, Haqqi TM. Epigallocathechin-3-gallate selectively inhibits interleukin-1β-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J Orthop Res. 2003. 21:102–109.32. Sugita-Konishi Y, Hara-Kudo Y, Amano F, Okubo T, Aoi N, Iwaki M, Kumagai S. Epigallocatechin gallate and gallocatechin gallate in green tea catechins inhibit extracellular release of Vero toxin from enterohemorrhagic Escherichia coli O157:H7. Biochim Biophys Acta. 1999. 1472:42–50.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Epigallocatechin-3-Gallate on the Expression of TGF-beta1, PKC alpha/betaII, and NF-kappaB in High-Glucose-Stimulated Glomerular Epithelial Cells
- Induction of Various Pro-inflammatory Mediators and Their Receptors in Human Astrocytoma Cell Line Stimulated by beta-amyloid Protein
- Nuclear Factor-kappa B Activation and Chemokine Genes Expression in HT-29 Intestinal Epithelial Cells in Response to Clostridium difficile Toxin A Stimulation
- Epigallocatechin-3-gallate Inhibits the Expression of Adhesion Molecules by Blocking Nuclear Factor Kappa B Signaling in Intestinal Epithelial Cells
- TNF-alpha-induced Increase of Human beta-defensin-2 Expression in HaCaT Cell Lines