Tuberc Respir Dis.
2006 Jun;60(6):663-672. 10.4046/trd.2006.60.6.663.
Particulate Matter from Asian Dust Storms Induces the Expression of Proinflammatory Cytokine in A549 Epithelial Cells
- Affiliations
-
- 1Division of Pulmonary Medicine, Department of Internal Medicine, Gachon Medical School Gil Medical Center, Incheon, Korea. jsw@gilhospital.com
- KMID: 1630805
- DOI: http://doi.org/10.4046/trd.2006.60.6.663
Abstract
-
BACKGROUND: PM10(Particulate matter with a diameter < 10micrometer), which is characterized by different environmental conditions, is a complex mixture of organic and inorganic compounds. The Asian dust event caused by meteorological phenomena can also produce unique particulate matter in affected areas. This study investigated the cytokine produced by A549 epithelial cells exposed to particles collected during both the Asian dust pfenomenon and ambient air particles in a non-dusty period.
METHOD: Air samples were collected using a high volume air sampler(Sibata Model HV500F) with an air flow at 500l/min for at least 6 hours. The cytokine messenger RNA(mRNA) was measured using a reverse transcriptase polymerase chain reaction(RT-PCR). The A549 cells were exposed to 10 to 500microgram/microliter of a suspension containing PM10 for 24 hours. Each was compared with those in the non-exposed control cells.
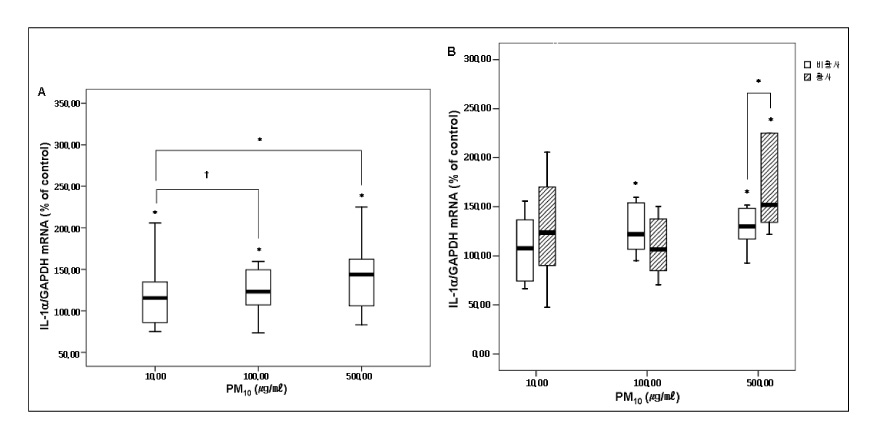
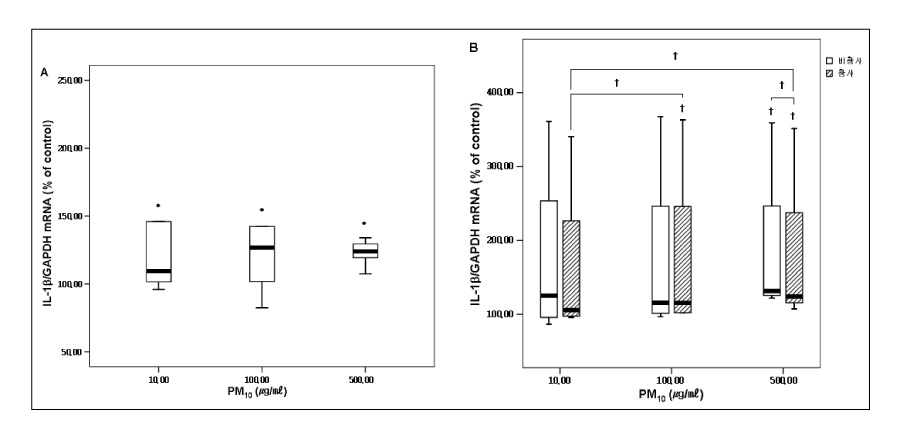
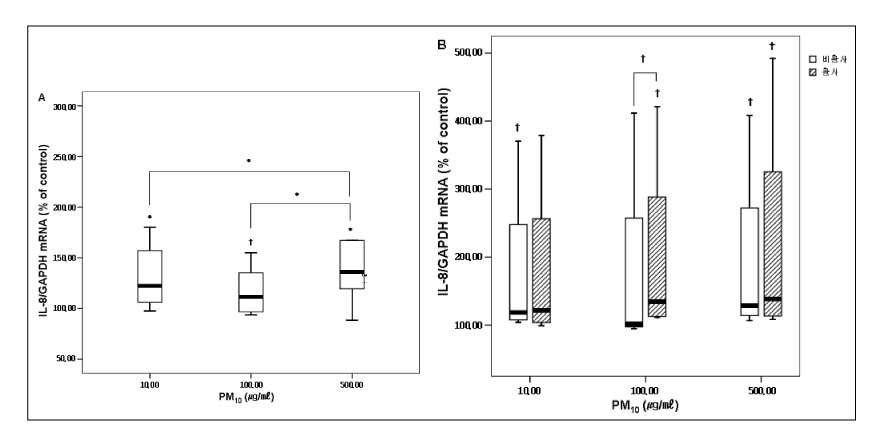
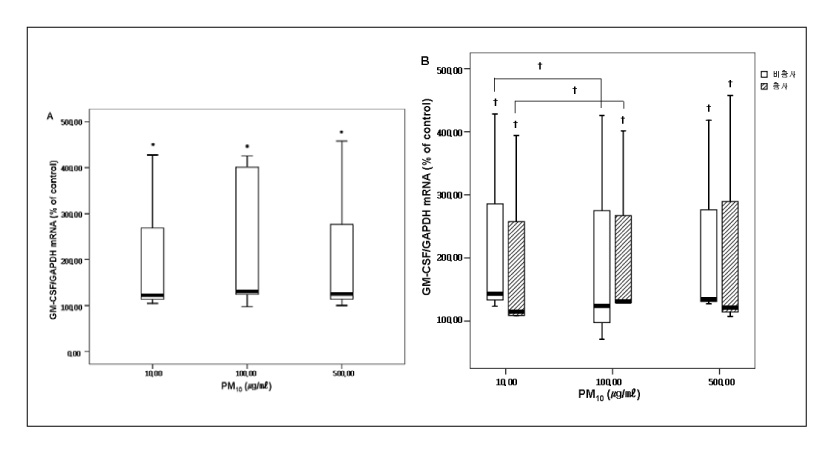
RESULT: The mRNA levels of interleukin(IL)-1alpha, IL-Ibeta , IL-8, and the granulocyte macrophage colony stimulating factor(GM-CSF) increased after veing exposed to PM10 in the ambient air particles, compared with those in the non-exposed control cells. The increase in IL-1alpha and IL-8 were dose dependent at a PM10 concentration between 100microgram/microliter and 500microgram/microliter. The mRNA level of IL-8 in the A549 epithelial cells was higher during the in the Asian dust period(500microgram/microliter) than during the non dust period.
CONCLUSION
A549 cells exposed to the PM10 collected during the Asian dust period produce more proinflammatory cytokine than during non-dusty period. This cytokine enhances the local inflammatory response in the airways and can also contribute to the systemic component of this inflammatory process.
MeSH Terms
Figure
Cited by 2 articles
-
Improvement of Atopic Dermatitis Severity after Reducing Indoor Air Pollutants
Hye One Kim, Jin Hye Kim, Soo Ick Cho, Bo Young Chung, In Su Ahn, Cheol Heon Lee, Chun Wook Park
Ann Dermatol. 2013;25(3):292-297. doi: 10.5021/ad.2013.25.3.292.Asian Sand Dust Up-Regulates MUC4 Expression in Human Upper Airway Epithelial Cells
Chang-Hwi Park, Yoo Sun Song, Chang Hoon Bae, Yoon Seok Choi, Si-Youn Song, Kyeong-Cheol Shin, Hyun Jung Jin, Yong-Dae Kim
Korean J Otorhinolaryngol-Head Neck Surg. 2017;60(5):222-231. doi: 10.3342/kjorl-hns.2016.17594.
Reference
-
1. Chun YS, Lim JY, Choi BC. The Features of Aerosol in Seoul by Asian Dust and Haze during Springtime from 1998 to 2002. Korean Meteorological Society. 2003. 39(4):459–474.2. Donaldson K, Stone V, Clouter A, Ren wick L, MacNee W. Ultrafine particle. Occup Environ Med. 2001. 58(3):211–215.3. Logan WP. Mortality in the London fog incident. 1952. Lancet. 1953. I:336–338.4. Saric M, Fugas M, Hrustic O. Effects of urban air pollution on school-age children. Arch Envirion Health. 1981. 36:101–108.5. Ware JH, Ferris BG, Dockery DW, Spengler JD, Stram DO, Speizer FE. Effects of ambient sulfar dioxides and suspended particles on respiratory health of preadolescent children. Am Rev Respir Dis. 1986. 133:834–842.6. Euler GL, Abbey DE, Magie AR, Hodgkin JE. Chronic obstructive pulmonary disease symptom effects of longterm cumulative exposure to ambient levels of total suspended particulates and dioxide in California seventh-day adventist resident. Arch Environ Health. 1987. 42:213–222.7. Ponka A, Virtanen M. Chronic bronchitis, emphysema, and low-level air pollution in Helsinki, 1978-1989. Environ Res. 1994. 65:207–217.8. Cohen CA, Hudson AR, Clausen JL, Knelson JH. Respiratory symptoms, spirometry and oxidant air pollution in non-smoking adults. Am Rev Respir Dis. 1972. 105:251–261.9. Pope CA III, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW Jr. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995. 151:669–674.10. Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis. 1993. 147:826–831.11. Dokery DW, Pope CA III. Acute respiratory effects of particulate air pollution. Ann Rev Public Health. 1994. 15:107–132.12. Choi KU, Paek DM. Asthma and Air Pollution in Korea. Korean Journal of Epidemiology. 1995. 17:64–75.13. Leem JH, Lee JT, Kim DG, Shin DC, Roh JH. Short-term Effects of Air Pollution on Hospital Visits for Respiratory Disease in Seoul. Korean Journal of Occupational and Environmental Medicine. 1998. 10(3):333–342.14. Park JU, Lim YH, Kyung SY, An CH, Lee SP, Jeong SH, Ju YS. Effects of Ambient Particulate Matter (PM10) on Peak Expiratory Flow and Respiratory Symptoms in Subjects with Bronchial Asthma During Yellow Sand Period. Tuberculosis and Respiratory Disease. 2003. 55(6):570–578.15. Carter JD, Ghio AJ, Samet JM, Devlin RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicol Appl Pharmacol. 1997. 146:180–188.16. Kennedy T, Ghio AJ, Reed W, Samet J, Zagorski J, Quay J, Carter J, Dailey L, Hoidal JR, Devlin RB. Copper-dependent inflammation and nuclear factor-kappaB activation by particulate air pollution. Am J Respir Cell Mol Biol. 1998. 19:366–378.17. Becker S, Soukup JM, Gilmour MI, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996. 141:637–648.18. Mills PR, Davies RJ, Devalia JL. Airway epithelial cells, cytokines, and pollutants. Am J Respir Crit Care Med. 1999. 160:S38–S43.19. Platzer E. Human hemopoietic growth factors. Eur J Haematol. 1989. 42:1–15.20. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999. 340:448–454.21. Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, Bosia A, Marchisio PC, Mantovani A. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991. 87:986–995.22. Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996. 153:530–534.23. van Pelt LJ, Huisman MV, Weening RS, von dem Borne AE, Roos D, van Oers RH. A single dose of granulocyte-macrophage colony-stimulating factor induces systemic interleukin-8 release and neutrophil activation in healthy volunteers. Blood. 1996. 87:5305–5313.24. Lopez AF, Williamson DJ, Gamble JR, Begley CG, Harlan JM, Klebanoff SJ, Waltersdorph A, Wong G, Clark SC, Vadas MA. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986. 78:1220–1228.25. Terashima T, Wiggs B, English D, Hogg JC, van Eeden SF. Phagocytosis of small carbon particles by alveolar macrophages stimulates the release of PMN from the bone marrow. Am J Respir Crit Care Med. 1997. 155:1441–1447.26. Tan WC, Qiu D, Liam BL, Ng TP, Lee SH, van Eeden SF, D'Yachkova Y, Hogg JC. The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med. 2000. 161:1213–1217.27. Mukae H, Hogg JC, English D, Vincent R, van Eeden SF. Phagocytosis of particulate air pollutants by human alveolar macrophages stimulates the bone marrow. Am J Physiol. 2000. 279:L924–L931.28. Fujii Takeshi, Hayashi Shizu, Hogg James C, Vincent Renaud, Van Eeden Stephan F. Particulate Matter Induces Cytokine Expression in Human Bronchial Epithelial Cells. Am J Respir Cell Mol Biol. 2001. 25:265–271.29. Boland S, Baeza-Squiban A, Fournier T, Houcine O, Gendron MC, Chevrier M, Jouvenot G, Coste A, Aubier M, Marano F. Diesel exhaust particles are taken up by human airway epithelial cells in vitro and alter cytokine production. Am J Physiol. 1999. 276:L604–L613.30. Osornio-Vargar AR, Bonner JC, Alfaro-Moreno E, Martinez L, Garcia-Cueller C, Ponce-de-Leon Rosales S, Miranda J, Rosas I. proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ Health Perspect. 2003. 111(10):1289–1293.31. Long CM, Suh HH, Kobzik L, Catalano PJ, Ning YY, Koutrakis P. A pilot investigation of the relative toxicity of indoor and outdoor fine particlesin;in vitro effects of endotoxin and other particulate properties. Environ Health Perspect. 2001. 109(10):1019–1026.32. van Eeden SF, Kitagawa Y, Sato Y, Hogg JC. Polymorphonuclear leukocytes released from the bone marrow and acute lung injury. Chest. 1999. 116(1):Suppl. S43–S46.33. Adamson IY, Prieditis H, Vincent R. Pulmonary toxicity of an atmospheric particulate sample is due to the soluble fraction. Toxicol Appl Pharmacol. 1999. 157:43–50.34. Baak YM, Kim JH, Kim KA, Ro CU, Kim HJ, Lim Y. Effects of Particulate Matters on A549 and RAW 264.7 Cells. Korean J Prev Med. 2001. 34:41–46.35. Chung YS, Kim HS, Park KH, Jhun JG, Chen SJ. Atmospheric Loadings , Concentrations and Visibility Associated with Yellow Sand Observed in 1997-2000 : Satellite and Meteorological Analysis. Korean Meteorological Society. 2000. 36(5):583–600.36. Kim MY, Cho SJ, Kim KR, Lee MH. The Behaviour of Dust Concentrations During Sand Storm in Seoul Area. Jour. Korean Earth Science Society. 2003. 24(4):315–324.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Particulate Matter 10 from Asian Dust Storms Induces the Expression of Reactive Oxygen Species, NF-kappaB, TGF-beta and Fibronectin in WI-26 VA4 Epithelial Cells
- Asian Dust Particles Induce TGF-beta1 via Reactive Oxygen Species in Bronchial Epithelial Cells
- Effect of the Asian Dust Events on Respiratory Disease During the Spring
- The Effect of Particulate Matter 10 from Asian Dust on the Production of Reactive Oxygen Species, TGF-beta, NF-kappaB, PDGF-alpha and Fibronectin in MRC-5 Fibroblast Cells
- Effects of Particulate Matters on A549 and RAW 264.7 Cells