Immune Netw.
2014 Feb;14(1):45-53. 10.4110/in.2014.14.1.45.
Ursodeoxycholic Acid Ameliorates Pain Severity and Cartilage Degeneration in Monosodium Iodoacetate-Induced Osteoarthritis in Rats
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul 137-040, Korea.
- 2The Rheumatism Research Center, Catholic Research Institute of Medical Science, The Catholic University of Korea, Seoul 137-040, Korea. iammila@catholic.ac.kr
- 3Division of Hepatology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul 137-040, Korea.
- KMID: 1508828
- DOI: http://doi.org/10.4110/in.2014.14.1.45
Abstract
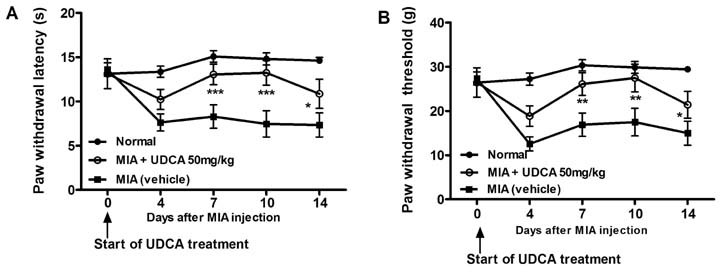
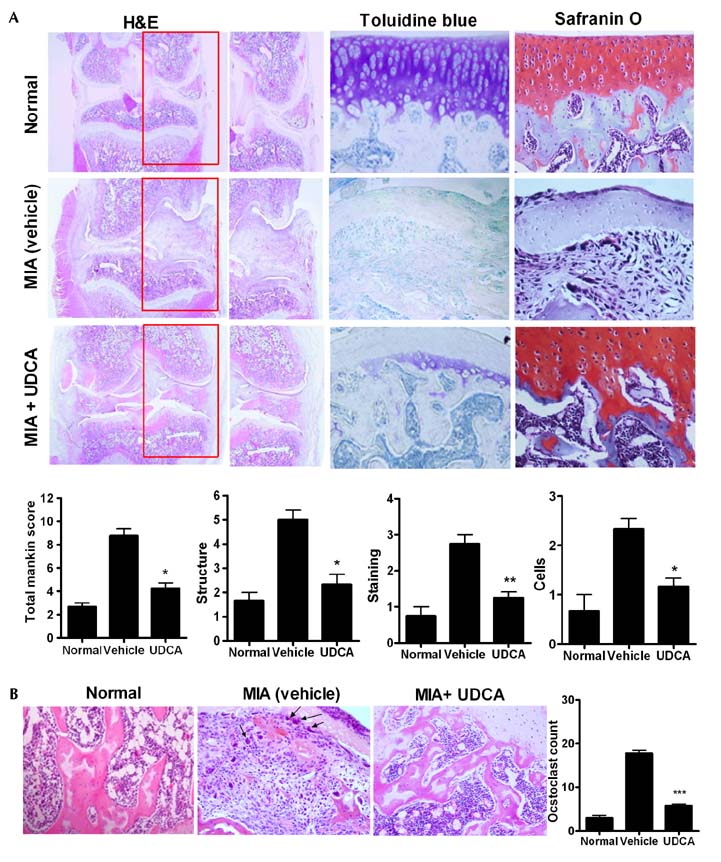
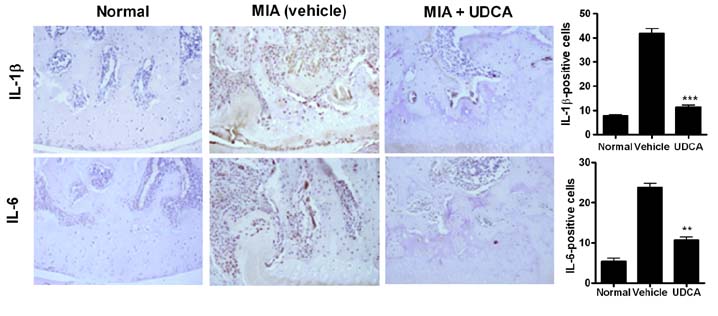
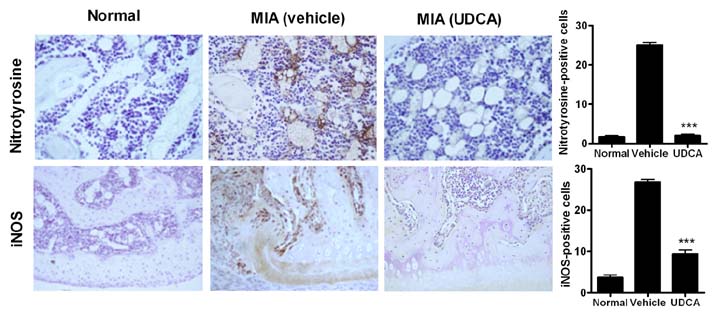
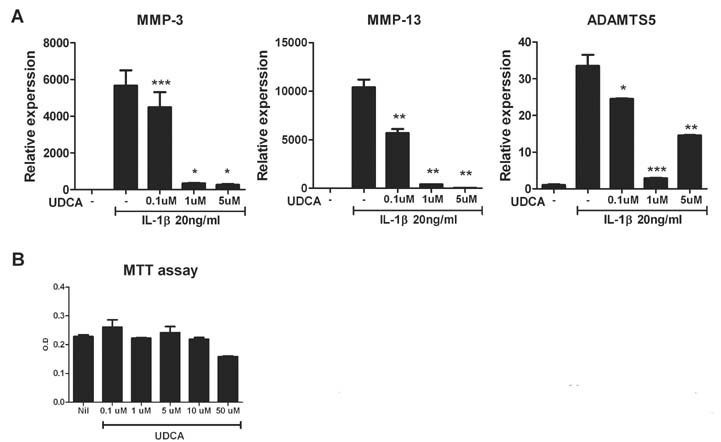
- Osteoarthritis (OA) is a degenerative joint disease characterized by a progressive loss of cartilage. And, increased oxidative stress plays a relevant role in the pathogenesis of OA. Ursodeoxycholic acid (UDCA) is a used drug for liver diseases known for its free radical-scavenging property. The objectives of this study were to investigate the in vivo effects of UDCA on pain severity and cartilage degeneration using an experimental OA model and to explore its mode of actions. OA was induced in rats by intra-articular injection of monosodium iodoacetate (MIA) to the knee. Oral administration UDCA was initiated on the day of MIA injection. Limb nociception was assessed by measuring the paw withdrawal latency and threshold. Samples were analyzed macroscopically and histologically. Immunohistochemistry was used to investigate the expression of interleukin-1beta (IL-1beta), IL-6, nitrotyrosine and inducible nitric oxide synthase (iNOS) in knee joints. UDCA showed an antinociceptive property and attenuated cartilage degeneration. OA rats given oral UDCA significantly exhibited a decreased number of osteoclasts in subchondral bone legion compared with the vehicle-treated OA group. UDCA reduced the expression of IL-1beta, IL-6, nitrotyrosine and iNOS in articular cartilage. UDCA treatment significantly attenuated the mRNA expression of matrix metalloproteinase-3 (MMP-3), -13, and ADAMTS5 in IL-1beta-stimulated human OA chondrocytes. These results show the inhibitory effects of UDCA on pain production and cartilage degeneration in experimentally induced OA. The chondroprotective properties of UDCA were achieved by suppressing oxidative damage and inhibiting catabolic factors that are implicated in the pathogenesis of cartilage damage in OA.
MeSH Terms
-
Administration, Oral
Animals
Cartilage*
Cartilage, Articular
Chondrocytes
Extremities
Humans
Immunohistochemistry
Injections, Intra-Articular
Interleukin-1beta
Interleukin-6
Joint Diseases
Knee
Knee Joint
Liver Diseases
Nitric Oxide Synthase Type II
Nociception
Osteoarthritis*
Osteoclasts
Oxidative Stress
Rats*
RNA, Messenger
Ursodeoxycholic Acid*
Interleukin-1beta
Interleukin-6
Nitric Oxide Synthase Type II
RNA, Messenger
Ursodeoxycholic Acid
Figure
Reference
-
1. Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006; 354:841–848.2. Chadjichristos C, Ghayor C, Kypriotou M, Martin G, Renard E, Ala-Kokko L, Suske G, de Crombrugghe B, Pujol JP, Galera P. Sp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytes. J Biol Chem. 2003; 278:39762–39772.
Article3. Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990; 144:499–505.4. Remans PH, Wijbrandts CA, Sanders ME, Toes RE, Breedveld FC, Tak PP, van Laar JM, Reedquist KA. CTLA-4IG suppresses reactive oxygen species by preventing synovial adherent cell-induced inactivation of Rap1, a Ras family GTPASE mediator of oxidative stress in rheumatoid arthritis T cells. Arthritis Rheum. 2006; 54:3135–3143.
Article5. Najim RA, Sharquie KE, Abu-Raghif AR. Oxidative stress in patients with Behcet's disease: I correlation with severity and clinical parameters. J Dermatol. 2007; 34:308–314.
Article6. Scott JL, Gabrielides C, Davidson RK, Swingler TE, Clark IM, Wallis GA, Boot-Handford RP, Kirkwood TB, Taylor RW, Young DA. Superoxide dismutase downregulation in osteoarthritis progression and end-stage disease. Ann Rheum Dis. 2010; 69:1502–1510.
Article7. Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem. 2000; 275:20069–20076.
Article8. Mendes AF, Caramona MM, Carvalho AP, Lopes MC. Hydrogen peroxide mediates interleukin-1beta-induced AP-1 activation in articular chondrocytes: implications for the regulation of iNOS expression. Cell Biol Toxicol. 2003; 19:203–214.
Article9. Tiku ML, Allison GT, Naik K, Karry SK. Malondialdehyde oxidation of cartilage collagen by chondrocytes. Osteoarthritis Cartilage. 2003; 11:159–166.
Article10. Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, Currie MG, Martel-Pelletier J. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo : possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2000; 43:1290–1299.
Article11. Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997; 113:884–890.
Article12. Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012; 36:Suppl 1. S3–S12.
Article13. Lapenna D, Ciofani G, Festi D, Neri M, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Antioxidant properties of ursodeoxycholic acid. Biochem Pharmacol. 2002; 64:1661–1667.
Article14. Ljubuncic P, Fuhrman B, Oiknine J, Aviram M, Bomzon A. Effect of deoxycholic acid and ursodeoxycholic acid on lipid peroxidation in cultured macrophages. Gut. 1996; 39:475–478.
Article15. Lukivskaya O, Zavodnik L, Knas M, Buko V. Antioxidant mechanism of hepatoprotection by ursodeoxycholic acid in experimental alcoholic steatohepatitis. Adv Med Sci. 2006; 51:54–59.16. Kuettner KE, Pauli BU, Gall G, Memoli VA, Schenk RK. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. Isolation, culture characteristics, and morphology. J Cell Biol. 1982; 93:743–750.
Article17. Sharma AR, Jagga S, Lee SS, Nam JS. Interplay between Cartilage and Subchondral Bone Contributing to Pathogenesis of Osteoarthritis. Int J Mol Sci. 2013; 14:19805–19830.
Article18. Guzman RE, Evans MG, Bove S, Morenko B, Kilgore K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritis. Toxicol Pathol. 2003; 31:619–624.
Article19. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011; 7:33–42.
Article20. Kobayashi K, Imaizumi R, Sumichika H, Tanaka H, Goda M, Fukunari A, Komatsu H. Sodium iodoacetate-induced experimental osteoarthritis and associated pain model in rats. J Vet Med Sci. 2003; 65:1195–1199.
Article21. Chevalier X, Giraudeau B, Conrozier T, Marliere J, Kiefer P, Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005; 32:1317–1323.22. Sanghi D, Avasthi S, Mishra A, Singh A, Agarwal S, Srivastava RN. Is radiology a determinant of pain, stiffness, and functional disability in knee osteoarthritis? A cross-sectional study. J Orthop Sci. 2011; 16:719–725.
Article23. Cotofana S, Wyman BT, Benichou O, Dreher D, Nevitt M, Gardiner J, Wirth W, Hitzl W, Kwoh CK, Eckstein F, Frobell RB. Relationship between knee pain and the presence, location, size and phenotype of femorotibial denuded areas of subchondral bone as visualized by MRI. Osteoarthritis Cartilage. 2013; 21:1214–1222.
Article24. Dean DD, Azzo W, Martel-Pelletier J, Pelletier JP, Woessner JF Jr. Levels of metalloproteases and tissue inhibitor of metalloproteases in human osteoarthritic cartilage. J Rheumatol. 1987; 14 Spec No:43–44.25. Huebner JL, Otterness IG, Freund EM, Caterson B, Kraus VB. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis. Arthritis Rheum. 1998; 41:877–890.
Article26. Tanaka S, Hamanishi C, Kikuchi H, Fukuda K. Factors related to degradation of articular cartilage in osteoarthritis: a review. Semin Arthritis Rheum. 1998; 27:392–399.
Article27. Durigova M, Nagase H, Mort JS, Roughley PJ. MMPs are less efficient than ADAMTS5 in cleaving aggrecan core protein. Matrix Biol. 2011; 30:145–153.
Article28. Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007; 56:575–585.
Article29. Carlo MD Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003; 48:3419–3430.
Article30. Poupon RE, Balkau B, Eschwege E, Poupon R. UDCA-PBC Study Group. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. N Engl J Med. 1991; 324:1548–1554.
Article31. Corpechot C, Carrat F, Bahr A, Chretien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005; 128:297–303.
Article32. Okada K, Shoda J, Taguchi K, Maher KM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, Oda K, Warabi E, Ishii T, Osaka K, Hyodo I, Yamamoto M. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008; 295:G735–G747.
Article33. Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010; 690:12–23.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-osteoarthritis effect of Boswellia serrata gum resin extract in monosodium iodoacetate-induced osteoarthritic Sprague-Dawley rats
- Prevention or treatment of enzyme treated royal jelly on monosodium-iodoacetate-induced osteoarthritis
- Pathological Characteristics of Monosodium Iodoacetate-Induced Osteoarthritis in Rats
- Anti-inflammatory effect of egg white-chalcanthite and purple bamboo salts mixture on arthritis induced by monosodium iodoacetate in Sprague-Dawley rats
- Micro-CT Arthrographic Analysis of Monosodium Iodoacetate-Induced Osteoarthritis in Rat Knees