Korean J Physiol Pharmacol.
2012 Oct;16(5):305-312. 10.4196/kjpp.2012.16.5.305.
Effects of Treadmill Exercise on the Recovery of Dopaminergic Neuron Loss and Muscle Atrophy in the 6-OHDA Lesioned Parkinson's Disease Rat Model
- Affiliations
-
- 1College of Nursing, Seoul National University, Seoul 110-744, Korea.
- 2Department of Neuropsychiatry, Graduate School of Oriental Medicine, Dongguk University, Goyang 410-773, Korea.
- 3Department of Nursing, Cheongju University, Cheongju 360-764, Korea. antheresa@cju.ac.kr
- 4Dongguk University Research Institute of Biotechnology, Seoul 100-715, Korea. jsong0304@dongguk.edu
- KMID: 1493962
- DOI: http://doi.org/10.4196/kjpp.2012.16.5.305
Abstract
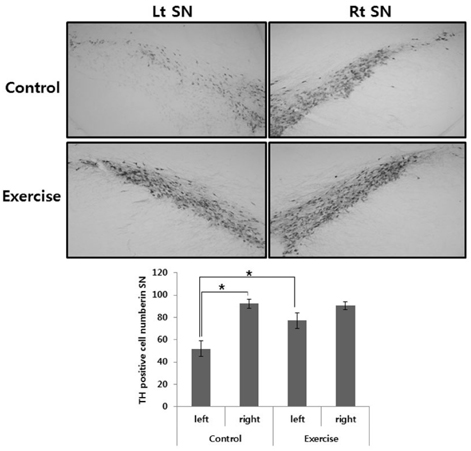
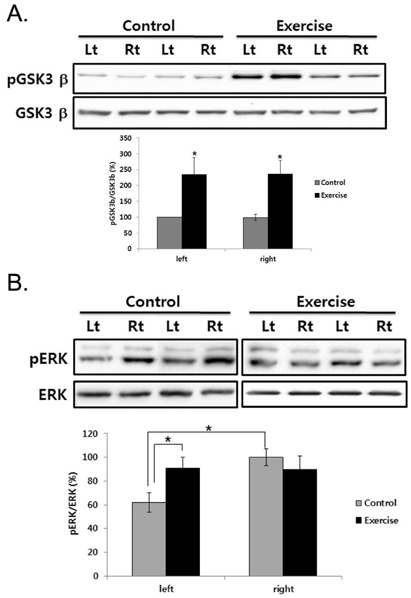
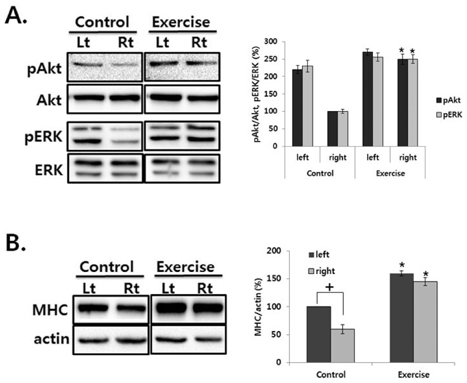
- This study was to determine the effect of exercise on the recovery of dopaminergic neuron loss and muscle atrophy in 6-OHDA-induced hemi Parkinson's disease model. Exercise was loaded twice per day for 30 minutes each time, at 5 days after 6-OHDA lesioning and continued for 16 days using a treadmill. Exercise significantly increased the number of tyrosine hydroxylase positive neuron in the lesioned substantia nigra and the expression level of tyrosine hydroxylase in the striatum compared with the control group. To examine which signaling pathways may be involved in the exercise, the phosphorylation of GSK3beta and ERK were observed in the striatum. In the control group, basal level of GSK3beta phosphorylation was less than in both striatum, but exercise increased it. ERK phosphorylation decreased in the lesioned striatum, but exercise recovered it. These findings suggest that exercise inactivates GSK3beta by phosphorylation which may be involved in the neuroprotective effect of exercise on the 6-OHDA-induced cell death. In the exercise group, weight, and Type I and II fiber cross-sectional area of the contralateral soleus significantly recovered and expression of myosin heavy chain and Akt and ERK phosphorylation significantly increased by exercise. These results suggest that exercise recovers Parkinson's disease induced dopaminergic neuron loss and contralateral soleus muscle atrophy.
Keyword
MeSH Terms
-
Animals
Atrophy
Cell Death
Dopaminergic Neurons
Glycogen Synthase Kinase 3
Muscle, Skeletal
Muscles
Muscular Atrophy
Myosin Heavy Chains
Neurons
Neuroprotective Agents
Oxidopamine
Parkinson Disease
Phosphorylation
Rats
Substantia Nigra
Tyrosine 3-Monooxygenase
Glycogen Synthase Kinase 3
Myosin Heavy Chains
Neuroprotective Agents
Oxidopamine
Tyrosine 3-Monooxygenase
Figure
Cited by 2 articles
-
Alteration of Striatal Tetrahydrobiopterin in Iron-Induced Unilateral Model of Parkinson's Disease
Bijay Aryal, Jin-Koo Lee, Hak Rim Kim, Hyung-Gun Kim
Korean J Physiol Pharmacol. 2014;18(2):129-134. doi: 10.4196/kjpp.2014.18.2.129.Regulatory B Subunits of Protein Phosphatase 2A Are Involved in Site-specific Regulation of Tau Protein Phosphorylation
Un Young Yu, Byong Chul Yoo, Jung-Hyuck Ahn
Korean J Physiol Pharmacol. 2014;18(2):155-161. doi: 10.4196/kjpp.2014.18.2.155.
Reference
-
1. Sethi KD. Clinical aspects of Parkinson disease. Curr Opin Neurol. 2002. 15:457–460.2. Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Characteristics of parkinsonian and ataxic gaits: a study using surface electromyograms, angular displacements and floor reaction forces. J Neurol Sci. 2000. 174:22–39.3. Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996. 50:275–331.4. Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz GA, Pellis SM. Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson's disease (PD) reveals homology to deficits in animal models. Behav Brain Res. 2002. 133:165–176.5. Muir GD, Whishaw IQ. Ground reaction forces in locomoting hemi-parkinsonian rats: a definitive test for impairments and compensations. Exp Brain Res. 1999. 126:307–314.6. Cano-de-la-Cuerda R, Pérez-de-Heredia M, Miangolarra-Page JC, Muñoz-Hellín E, Fernández-de-Las-Peñas C. Is there muscular weakness in Parkinson's disease? Am J Phys Med Rehabil. 2010. 89:70–76.7. Kim Y, Choe MA. Effect of decreased locomotor activity on hindlimb muscles in a rat model of Parkinson's disease. J Korean Acad Nurs. 2010. 40:580–588.8. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999. 96:13427–13431.9. Tillerson JL, Caudle WM, Reverón ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003. 119:899–911.10. Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002. 934:1–6.11. Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003. 117:1037–1046.12. Yasuhara T, Hara K, Maki M, Matsukawa N, Fujino H, Date I, Borlongan CV. Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience. 2007. 149:182–191.13. Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008. 23:631–640.14. Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967. 50:197–218.15. Bigard XA, Janmot C, Merino D, Lienhard F, Guezennec YC, D'Albis A. Endurance training affects myosin heavy chain phenotype in regenerating fast-twitch muscle. J Appl Physiol. 1996. 81:2658–2665.16. Frimel TN, Kapadia F, Gaidosh GS, Li Y, Walter GA, Vandenborne K. A model of muscle atrophy using cast immobilization in mice. Muscle Nerve. 2005. 32:672–674.17. Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003. 9:344–350.18. Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005. 37:1974–1984.19. Nallegowda M, Singh U, Handa G, Khanna M, Wadhwa S, Yadav SL, Kumar G, Behari M. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson's disease: a pilot study. Am J Phys Med Rehabil. 2004. 83:898–908.20. Inkster LM, Eng JJ, MacIntyre DL, Stoessl AJ. Leg muscle strength is reduced in Parkinson's disease and relates to the ability to rise from a chair. Mov Disord. 2003. 18:157–162.21. Pääsuke M, Ereline J, Gapeyeva H, Joost K, Mõttus K, Taba P. Leg-extension strength and chair-rise performance in elderly women with Parkinson's disease. J Aging Phys Act. 2004. 12:511–524.22. Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007. 78:678–684.23. Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, Chavret F, Hetherington V, Baker K, Lim I. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007. 78:134–140.24. Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson's disease. Arch Phys Med Rehabil. 2003. 84:1109–1117.25. Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord. 2006. 21:1444–1452.26. Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003. 58:176–180.27. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002. 25:295–301.28. Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003. 28:1757–1769.29. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003. 14:125–130.30. Yi SS, Hwang IK, Yoo KY, Park OK, Yu J, Yan B, Kim IY, Kim YN, Pai T, Song W, Lee IS, Won MH, Seong JK, Yoon YS. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2009. 34:1039–1046.31. Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009. 1288:105–115.32. Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I. Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res. 2010. 1310:200–207.33. Ortega F, Pérez-Sen R, Morente V, Delicado EG, Miras-Portugal MT. P2X7, NMDA and BDNF receptors converge on GSK3 phosphorylation and cooperate to promote survival in cerebellar granule neurons. Cell Mol Life Sci. 2010. 67:1723–1733.34. Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004. 11:172–178.35. Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004. 18:1162–1164.36. Yong Y, Ding H, Fan Z, Luo J, Ke ZJ. Lithium fails to protect dopaminergic neurons in the 6-OHDA model of Parkinson's disease. Neurochem Res. 2011. 36:367–374.37. Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, You Y, Han Y, Chen R, Wang Y, Li M. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007. 52:1678–1684.38. Petit-Paitel A, Brau F, Cazareth J, Chabry J. Involvment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. PLoS One. 2009. 4:e5491.39. Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. Eur J Neurosci. 2011. 33:1264–1274.40. Connor B. Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat. Clin Exp Pharmacol Physiol. 2001. 28:896–900.41. Haavik J, Toska K. Tyrosine hydroxylase and Parkinson's disease. Mol Neurobiol. 1998. 16:285–309.42. Ziegler MG, Szechtman H. Relation between motor asymmetry and direction of rotational behaviour under amphetamine and apomorphine in rats with unilateral degeneration of the nigrostriatal dopamine system. Behav Brain Res. 1990. 39:123–133.43. Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav Brain Res. 2005. 162:1–10.44. Solomon AM, Bouloux PM. Modifying muscle mass-the endocrine perspective. J Endocrinol. 2006. 191:349–360.45. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001. 3:1014–1019.46. Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001. 3:1009–1013.47. McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004. 119:907–910.48. Price SR. Increased transcription of ubiquitin-proteasome system components: molecular responses associated with muscle atrophy. Int J Biochem Cell Biol. 2003. 35:617–628.49. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004. 117:399–412.50. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004. 14:395–403.51. Shi H, Scheffler JM, Zeng C, Pleitner JM, Hannon KM, Grant AL, Gerrard DE. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol. 2009. 296:C1040–C1048.52. Choe MA, Go JJ, Kwak HK, Baek J, Jung JY, Song YJ, An GJ. Comparison of hypertrophic effects of low-intensity exercise on rat hindlimb muscles between every other day exercise and everyday exercise. J Korean Biol Nurs Sci. 2011. 13:1–7.53. Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990. 68:1–12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphological Changes in Dopaminergic Neurons in Selective Parkinson's Rat Model
- Effects of Gypenosides on Dopaminergic Neuronal Cell Death in 6-Hydroxydopamine-lesioned Rat Model of Parkinson's Disease with Long-term L-DOPA Treatment
- Subthalamic Lesion Protects Nigral Dopaminergic Neurons from 6-Hydroxydopamine-induced Cytotoxicity in the Rat Model of Early Parkinson's Disease
- Effect of DHEA on Recovery of Muscle Atrophy Induced by Parkinson's Disease
- Effect of Decreased Locomotor Activity on Hindlimb Muscles in a Rat Model of Parkinson's Disease