Nat Prod Sci.
2016 Sep;22(3):187-192. 10.20307/nps.2016.22.3.187.
Effects of Gypenosides on Dopaminergic Neuronal Cell Death in 6-Hydroxydopamine-lesioned Rat Model of Parkinson's Disease with Long-term L-DOPA Treatment
- Affiliations
-
- 1College of Pharmacy and Research Center for Bioresource and Health, Chungbuk National University, Cheongju, Chungbuk 28644, Korea. myklee@chungbuk.ac.kr
- 2Department of Food and Nutrition, Chungcheong University, 38, Wuelgok-gil, Cheongju, Chungbuk 28171, Korea.
- KMID: 2355144
- DOI: http://doi.org/10.20307/nps.2016.22.3.187
Abstract
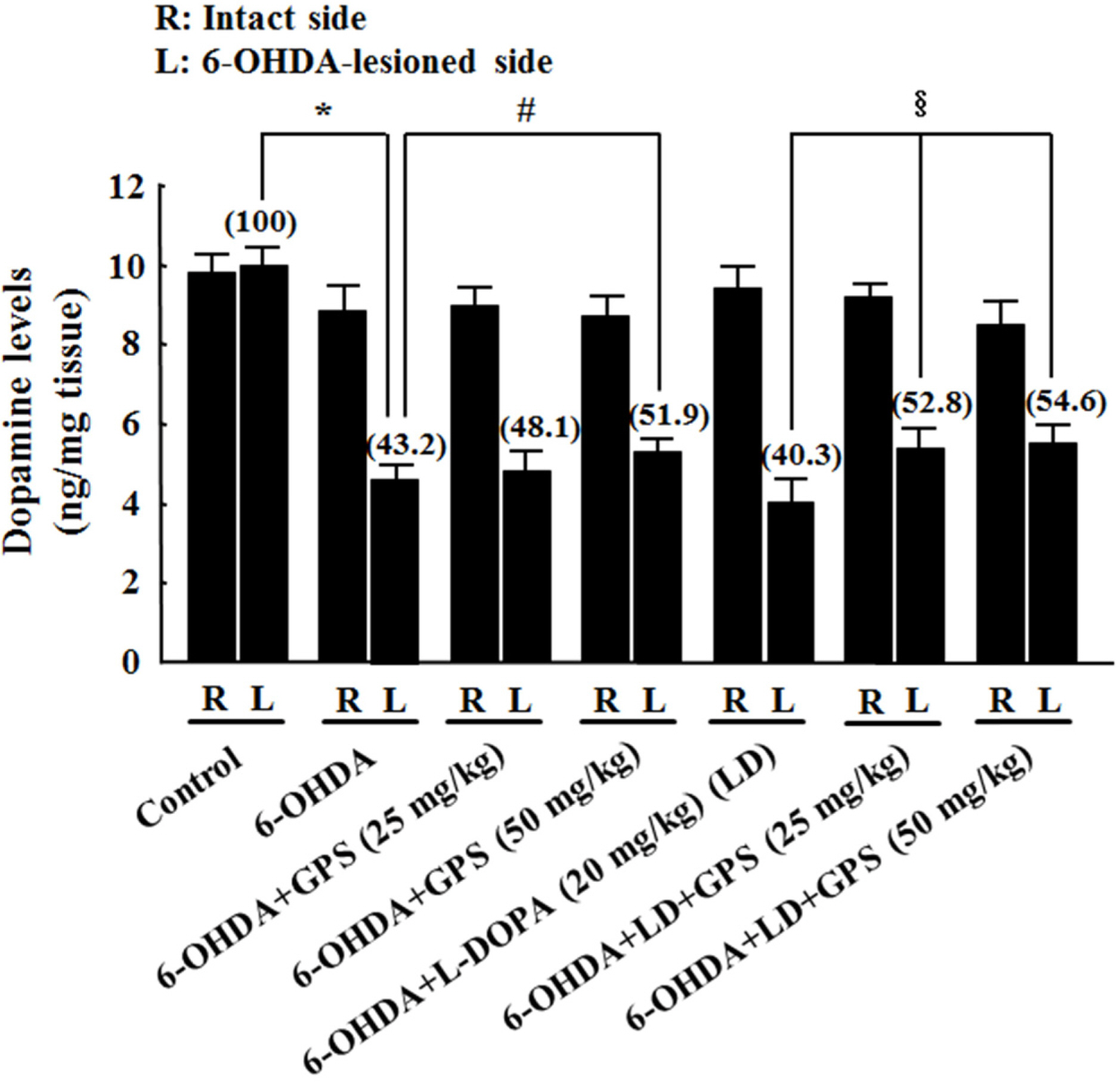
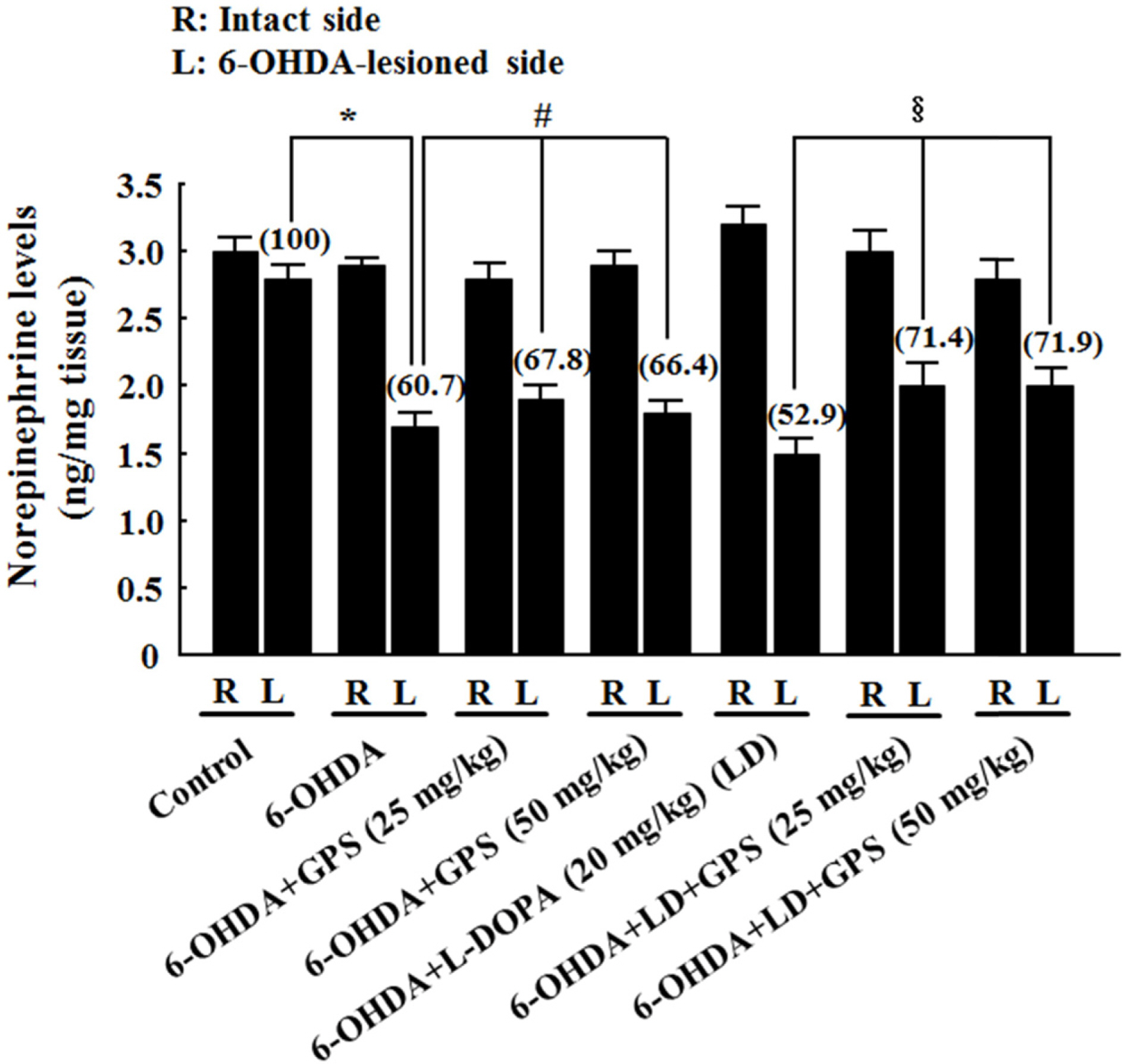
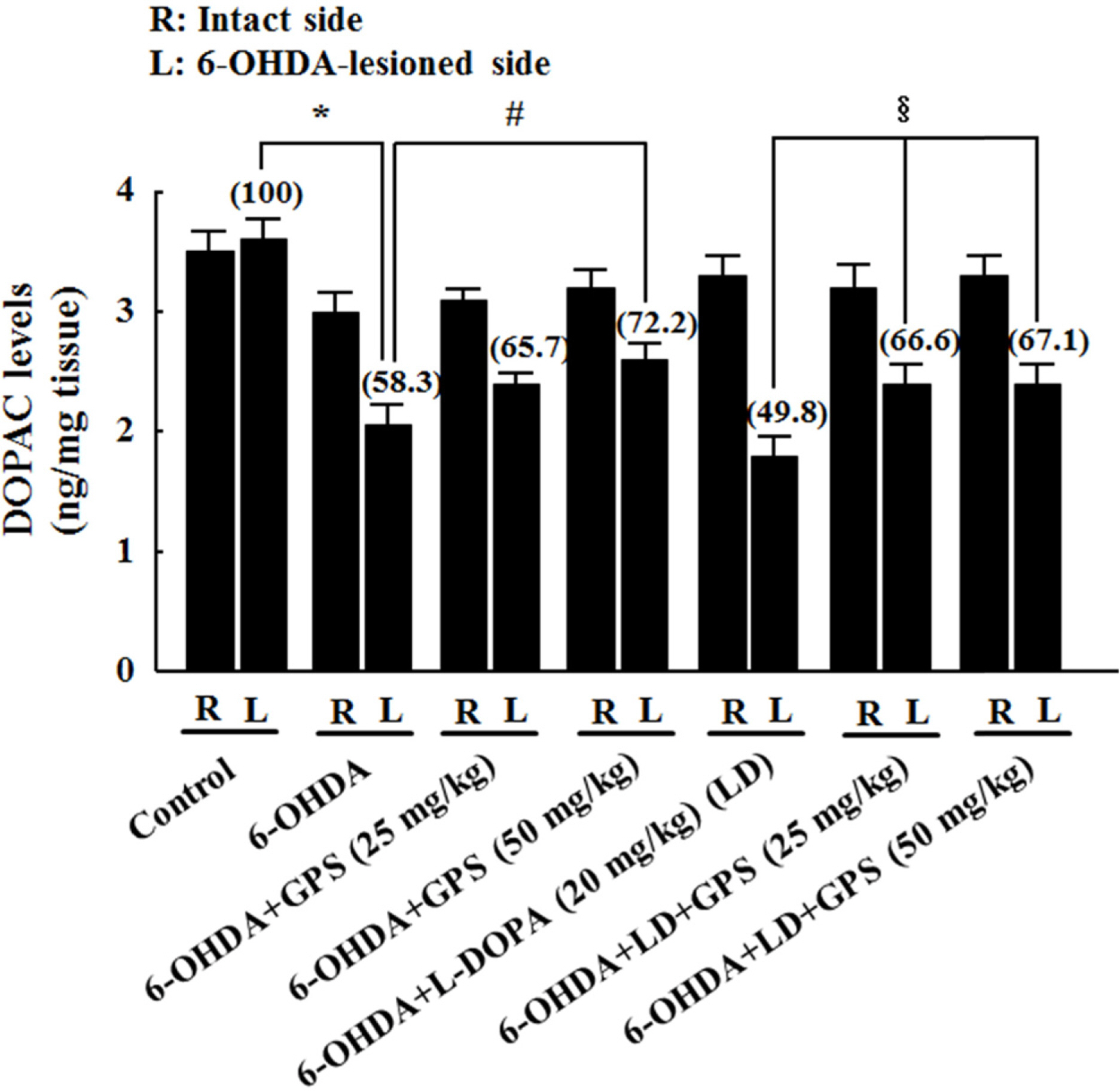
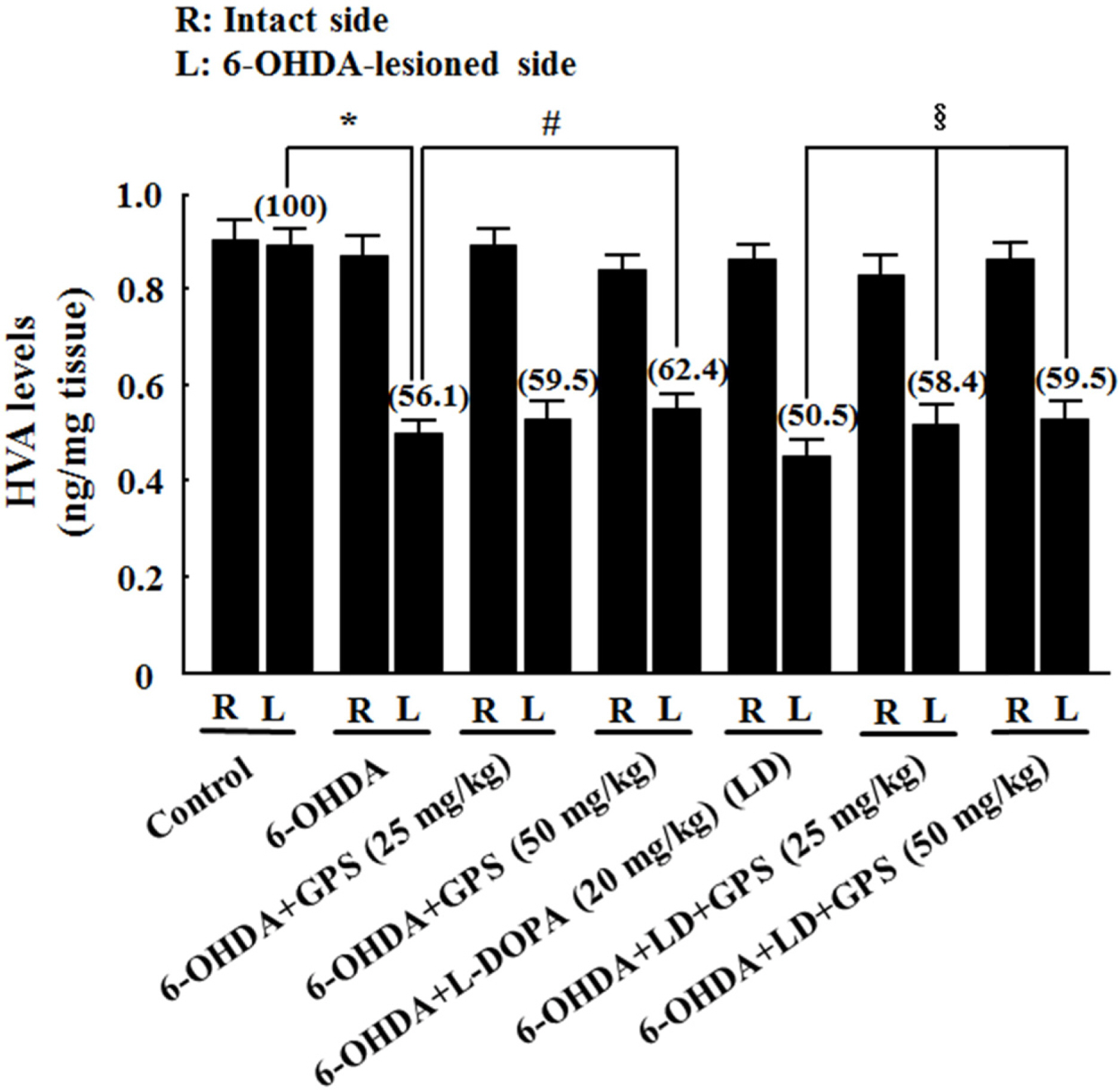
- The goal of this study was to determine whether gypenosides (GPS) exert protective effects against dopaminergic neuronal cell death in a 6-hydroxydopamine (OHDA)-lesioned rat model of Parkinson's disease (PD) with or without long-term 3,4-dihydroxyphenylalanine (L-DOPA) treatment. Rats were injected with 6-OHDA in the substantia nigra to induce PD-like symptoms; 14 days after injection, groups of 6-OHDA-lesioned animals were treated for 21 days with GPS (25 or 50 mg/kg) and/or L-DOPA (20 mg/kg). Dopaminergic neuronal cell death was assessed by counting tyrosine hydroxylase (TH)-immunopositive cells in the substantia nigra and measuring levels of dopamine, norepinephrine, 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) in the striatum. Dopaminergic neuronal cell death induced by 6-OHDA lesions was ameliorated by GPS treatment (50 mg/kg). L-DOPA treatment exacerbated 6-OHDA-induced dopaminergic neuronal cell death; however, these effects were partially reversed by GPS treatment (25 and 50 mg/kg). These results suggest that GPS treatment is protective against dopaminergic neuronal cell death in a 6-OHDA-lesioned rat model of PD with long-term L-DOPA treatment. Therefore, GPS may be useful as a phytotherapeutic agent for the treatment of PD.
Keyword
MeSH Terms
-
3,4-Dihydroxyphenylacetic Acid
Animals
Cell Death*
Dihydroxyphenylalanine
Dopamine
Dopaminergic Neurons*
Homovanillic Acid
Levodopa*
Models, Animal*
Norepinephrine
Oxidopamine
Parkinson Disease*
Rats*
Substantia Nigra
Tyrosine 3-Monooxygenase
3,4-Dihydroxyphenylacetic Acid
Dihydroxyphenylalanine
Dopamine
Homovanillic Acid
Levodopa
Norepinephrine
Oxidopamine
Tyrosine 3-Monooxygenase
Figure
Reference
-
(1). Fearnley J. M., Lees A. J.Brain. 1991; 114:2283–2301.(2). Fahn S. Ann. N. Y.Acad. Sci. 2003; 991:1–14.(3). Marsden C. D. J.Neurol. Neurosurg. Psychiatry. 1994; 57:672–681.(4). Jankovic J.Mov. Disord. 2005; 20:S11–S16.(5). Cheng N., Maeda T., Kume T., Kaneko S., Kochiyama H., Akaike A., Goshima Y., Misu Y.Brain Res. 1996; 16:278–283.(6). Walkinshaw G., Waters C. M. J.Clin. Invest. 1995; 95:2458–2464.(7). Shin K. S., Zhao T. T., Park K. H., Park H. J., Hwang B. Y., Lee C. K., Lee M. K.BMC Neurosci. 2015; 16:23.(8). Bové J., Perier C.Neuroscience. 2012; 211:51–76.(9). Jackson-Lewis V., Blesa J., Przedborski S.Parkinsonism Relat. Disord. 2012; 18:S183–S185.(10). Seidl S. E., Potashkin J. A.Front. Neurol. 2011; 2:68.(11). Razmovski-Naumovski V., Huang T. H. W., Tran V. H., Li G. Q., Duke C. C., Roufogalis B. D.Phytochem. Rev. 2005; 4:197–219.(12). Im S. A., Choi H. S., Hwang B. Y., Lee M. K., Lee C.K.Kor. J. Pharmacogn. 2009; 40:35–40.(13). Choi H. S., Zhao T. T., Shin K. S., Kim S. H., Hwang B. Y., Lee C. K., Lee M. K.Molecules. 2013; 18:4342–4356.(14). Choi H. S., Park M. S., Kim S. H., Hwang B. Y., Lee C. K., Lee M. K.Molecules. 2010; 15:2814–2824.(15). Shang L. S., Liu J. C., Zhu Q. J., Zhao L., Feng Y., Wang X., Cao W., Xin H.Brain Res. 2006; 1102:163–174.(16). Wang P., Niu L., Guo X. D., Gao L., Li W. X., Jia D., Wang X. L, Ma L. T., Gao G. D.Brain Res. Bull. 2010; 83:266–271.(17). Zhang G., Zhao Z., Gao L., Deng J., Wang B., Xu D., Liu B., Qu Y., Yu J., Li J., Gao G.Pharmacol. Biochem. Behav. 2011; 99:42–51.(18). Wang P., Niu L., Gao L., Li W. X., Jia D., Wang X. L., Gao G. D. J.Int. Med. Res. 2010; 38:1084–1092.(19). Paxinos G., Watson C.The rat brain in stereotaxic coordinates. 2nd ed. Academic Press;Australia: 1986.(20). Schwarting R. K. W., Huston J. P.Prog. Neurobiol. 1996; 50:275–331.(21). Mo J., Zhang H., Yu L. P., Sun P. H., Jin G. Z., Zhen X.Neurobiol. Aging. 2010; 31:926–936.(22). Izurieta-Sánchez P., Sarre S., Ebinger G., Michotte Y.Eur. J. Pharmacol. 1998; 353:33–42.(23). Ljungberg T., Ungerstedt U.Eur. J. Pharmacol. 1977; 46:147–151.(24). Blesa J., Phani S., Jackson-Lewis V., Przedborski S. J.BioMed. Biotechnol. 2012; 2012:845618.(25). Shin K. S., Zhao T. T., Choi H. S., Hwang B. Y., Lee C. K., Lee M. K.Brain Res. 2014; 1567:57–65.(26). Cenci M. A.Parkinsonism Relat. Disord. 2007; 13:S263–S267.(27). Cohen G.Neurotoxicology. 1984; 5:77–82.(28). Soto-Otero R., Méndez-Álvarez E., Hermida-Ameijeiras Á., Muñoz-Patiño A. M., Labandeira-Garcia J. L. J.Neurochem. 2000; 74:1605–1612.(29). Cadet J. L., Brannock C.Neurochem. Int. 1998; 32:117–131.(30). Fahn S., Cohen G.Ann. Neurol. 1992; 32:804–812. 2004.(31). Schober A.Cell Tissue Res. 2004; 318:215–224.(32). Blandini F., Armentero M. T., Martignoni E.Parkinsonism Relat. Disord. 2008; 14:S124–S129.(33). Severson J. A., Marcusson J., Winblad B., Finch C. E. J.Neurochem. 1982; 39:1623–1631.(34). Linert W., Herlinger E., Jameson R. F., Kienzl E., Jellinger K., Youdim M. B.Biochim. Biophys. Acta. 1996; 1316:160–168.(35). Maharaj H., Sukhdev Maharaj D., Scheepers M., Mokokong R., Daya S.Brain Res. 2005; 1063:180–186.(36). Basma A. N., Morris E. J., Nicklas W. J., Geller H. M. J.Neurochem. 1995; 64:825–832.(37). Migheli R., Godani C., Sciola L., Delogu M. R., Serra P. A., Zangani D., De Natale G., Miele E., Desole M. S. J.Neurochem. 1999; 73:1155–1163.(38). Zhang L., Dawson V. L., Dawson T. M.Pharmacol. Ther. 2006; 109:33–41.(39). Padovan-Neto F. E., Echeverry M. B., Tumas V., Del-Bel E. A.Neuroscience. 2009; 159:927–935.(40). Nicklas W. J., Vyas I., Heikkila R. E.Life Sci. 1985; 36:2503–2508.(41). Yacoubian T. A., Standaert D. G.Biochim. Biophys. Acta. 2009; 1792:676–687.(42). Cai T. S., Zhang S. F., Wang M. C.Chin. J. Clin. Rehabil. 2005; 9:106–107.(43). Tanner M. A., Bu X., Steimle J. A., Myers P. R.Nitric Oxide. 1999; 3:359–365.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphological Changes in Dopaminergic Neurons in Selective Parkinson's Rat Model
- Effects of Fetal Nondopaminergic Cortical Tissue Transplantation in the Rat Parkinsonian Model
- Neuroglial Reaction in the Substantia Nigra and Striatum of 6-Hydroxydopamine Induced Parkinson's Disease Rat Model
- The Behavioral Changes and the Patterns of Dopamine NeuronalDegeneration after Intrastriatal 6-hydroxydopamine Injection in Rat
- Tumor Necrosis Factor-Associated Protein 1 (TRAP1) is Released from the Mitochondria Following 6-hydroxydopamine Treatment