J Bacteriol Virol.
2008 Jun;38(2):53-60. 10.4167/jbv.2008.38.2.53.
Proteomic analysis of Helicobacter pylori J99 Outer Membrane Protein by Tandem Mass Spectrometry
- Affiliations
-
- 1Department of Microbiology,Gyeongsang National University College of Medicine, Jinju, Korea. scbaik@gaechuk.gsnu.ac.kr
- 2Department of Pediatrics, Gyeongsang National University College of Medicine, Jinju, Korea.
- 3Central Instrument Facility, Gyeongsang National University, Jinju, Korea.
- 4Research Institute of Life Science, Gyeongsang National University, Jinju, Korea.
- KMID: 1483975
- DOI: http://doi.org/10.4167/jbv.2008.38.2.53
Abstract
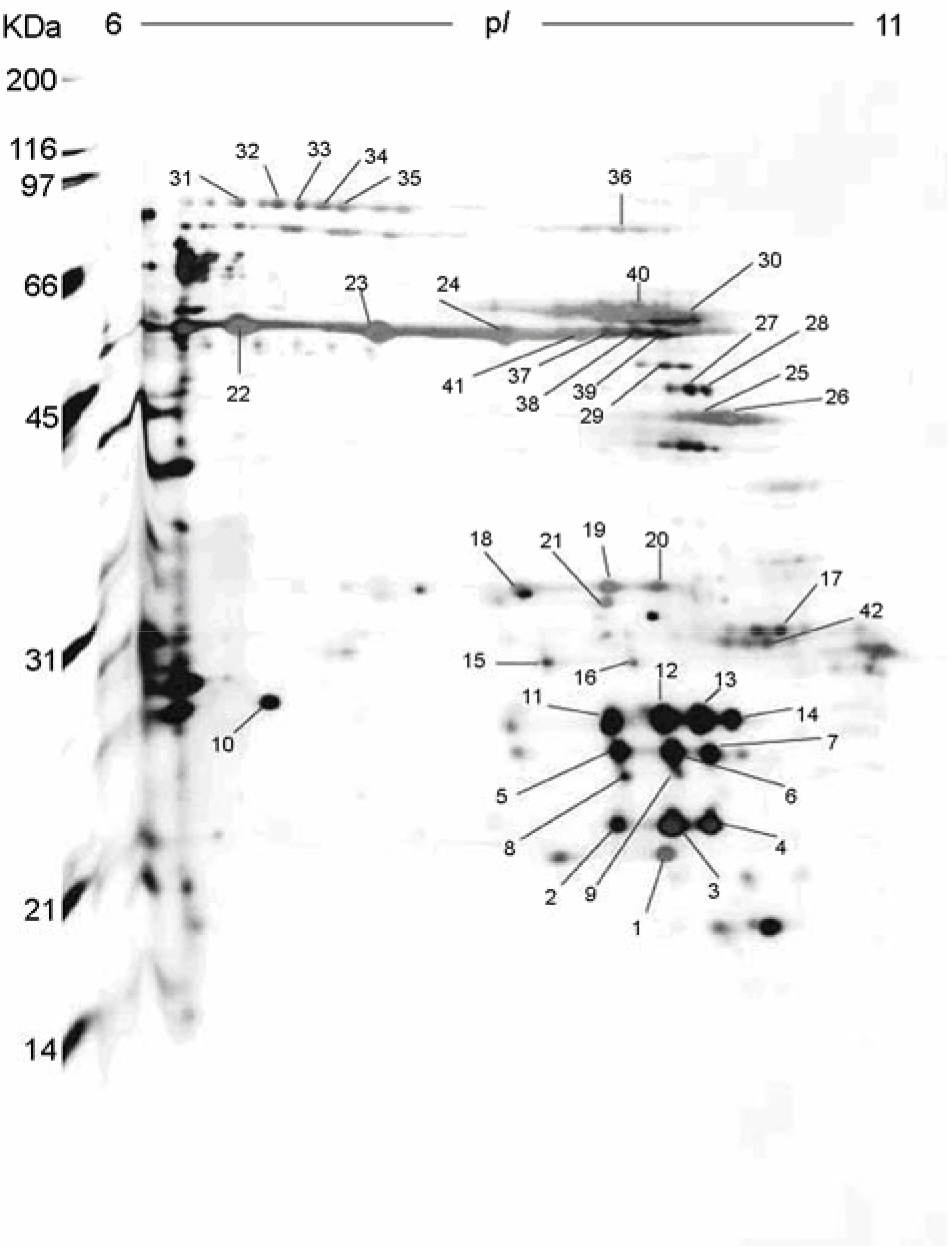
- The protein identity of sarcosine-insoluble outer membrane proteins (OMPs) of Helicobacter pylori J99 was determined with the basic study of understanding the function of proteins. A sarcosine-insoluble OMPs was resolved by two-dimensional electrophoresis with immobilized pH gradient strips. The most abundant proteins were shown in the alkaline pI regions (6.0~11.0) with molecular masses of 10 to 100 kDa. We have performed an extensive proteome analysis by quadrupole time of flight (Q-TOF) mass spectrometry (MS). Here, of 50 spots processed, 42 spots were identified, which represented 16 genes and we newly detected 8 kinds of proteins (JHP0119, JHP0388, JHP1046, JHP1405, JHP0073, JHP0551, JHP1382, JHP0552) from the sarcosin-insoluble fraction of H. pylori J99. Those may be used to elucidate the characterization of the OMPs of H. pylori J99, which will help identify new potential target proteins for vaccine development and drug therapy.
MeSH Terms
Figure
Cited by 1 articles
-
Helicobacter pylori: Bacterial Strategy for Incipient Stage and Persistent Colonization in Human Gastric Niches
Kwang-Ho Rhee, Jin-Sik Park, Myung-Je Cho
Yonsei Med J. 2014;55(6):1453-1466. doi: 10.3349/ymj.2014.55.6.1453.
Reference
-
References
1). Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, de Jonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 397:176–180. 1999.2). Baik SC, Kim KM, Song SM, Kim DS, Jun JS, Lee SG, Song JY, Park JU, Kang HL, Lee WK, Cho MJ, Youn HS, Ko GH, Rhee KH. Proteomic Analysis of sarcosine-insoluble outer membrane fraction of Helicobacter pylori strain 26695. J Bacteriol. 186:949–955. 2004.3). Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 18:298–305. 2002.
Article4). Blaser MJ. Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology. 93:371–383. 1987.5). Bumann D, Aksu S, Wendland M, Janek K, Zimny-Arndt U, Sabarth N, Meyer TF, Jungblut PR. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect Immun. 70:3396–3403. 2002.6). Cullen PA, Cordwell SJ, Bulach DM, Haake DA, Adler B. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect Immun. 70:2311–2318. 2002.7). Delany I, Pacheco AB, Spohn G, Rappuoli R, Scarlato V. Iron-dependent transcription of the frpB gene of Helicobacter pylori is controlled by the Fur repressor protein. J Bacteriol. 183:4932–4937. 2001.8). Exner MM, Doig P, Trust TJ, Hancock RE. Isolation and characterization of a family of porin proteins from Helicobacter pylori. Infect Immun. 63:1567–1572. 1995.9). Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 279:373–377. 1998.10). Kim N, Weeks DL, Shin JM, Scott DR, Young MK, Sachs G. Proteins released by Helicobacter pylori in vitro. J Bacteriol. 184:6155–6162. 2002.11). Maguire PB, Wynne KJ, Harney DF, O'Donoghue NM, Stephens G, Fitzgerald DJ. Identification of the phosphotyrosine proteome from thrombin activated platelets. Proteomics. 2:642–648. 2002.
Article12). Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadström T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarström L, Borén T. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 297:573–578. 2002.13). McGovern KJ, Blanchard TG, Gutierrez JA, Czinn SJ, Krakowka S, Youngman P. gamma-Glutamyltransferase is a Helicobacter pylori virulence factor but is not essential for colonization. Infect Immun. 69:4168–4173. 2001.14). Molloy MP, Herbert BR, Slade MB, Rabilloud T, Nouwens AS, Williams KL, Gooley AA. Proteomic analysis of the Escherichia coli outer membrane. Eur J Biochem. 267:2871–2881. 2000.15). Molloy MP, Phadke ND, Maddock JR, Andrews PC. Two-dimensional electrophoresis and peptide mass fingerprinting of bacterial outer membrane proteins. Electrophoresis. 22:1686–1696. 2001.
Article16). Odenbreit S, Till M, Hofreuter D, Faller G, Haas R. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol Microbiol. 31:1537–1548. 1999.17). Peck B, Ortkamp M, Diehl KD, Hundt E, Knapp B. Conservation, localization and expression of HopZ, a protein involved in adhesion of Helicobacter pylori. Nucleic Acids Res. 27:3325–3333. 1999.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of Helicobacter pylori Strain 51 Major Outer Membrane Proteins by Quadrupole Time of Flight Mass Spectrometry
- Proteomics Approach in Helicobacter pylori Researches
- Sporozoite proteome analysis of Cryptosporidium parvum by one-dimensional SDS-PAGE and liquid chromatography tandem mass spectrometry
- Comparison of Proteome Component of Helicobacter pylori in Different Atmospheric CO2 Concentration
- Identification of Leukemia Surface Proteins Using a Proteomic Technique