J Bacteriol Virol.
2008 Sep;38(3):97-107. 10.4167/jbv.2008.38.3.97.
Depressed CCL5 Expression in Human Pulmonary Tuberculosis
- Affiliations
-
- 1Infection Signaling Network Research Center, College of Medicine, Chungnam National University, Daejeon, Korea. hayoungj@cnu.ac.kr
- 2Department of Microbiology, College of Medicine, Konyang University, Daejeon, Korea.
- 3Department of Microbiology, College of Medicine, Chungnam National University, Daejeon, Korea.
- 4Internal Medicine, College of Medicine, Konyang University, Daejeon, Korea.
- KMID: 1483968
- DOI: http://doi.org/10.4167/jbv.2008.38.3.97
Abstract
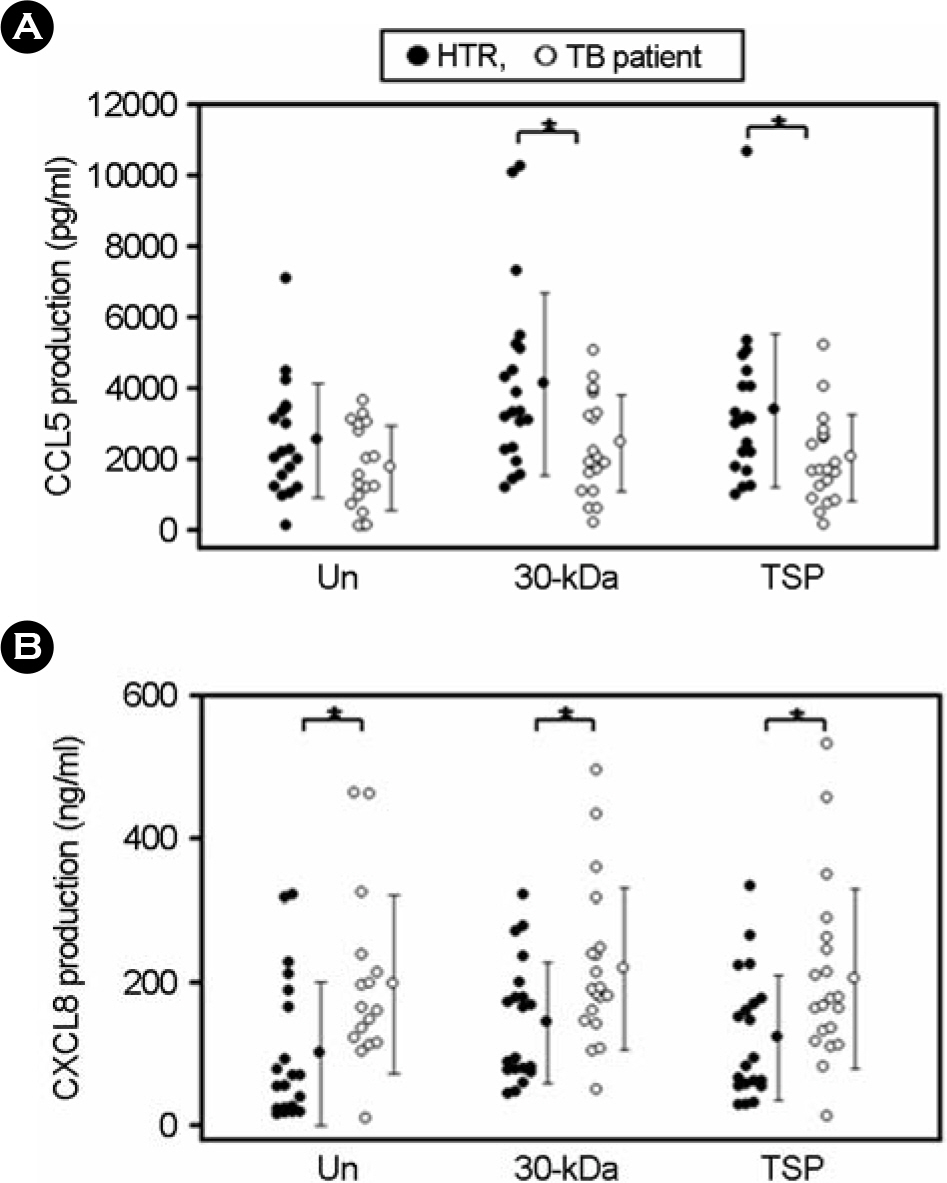
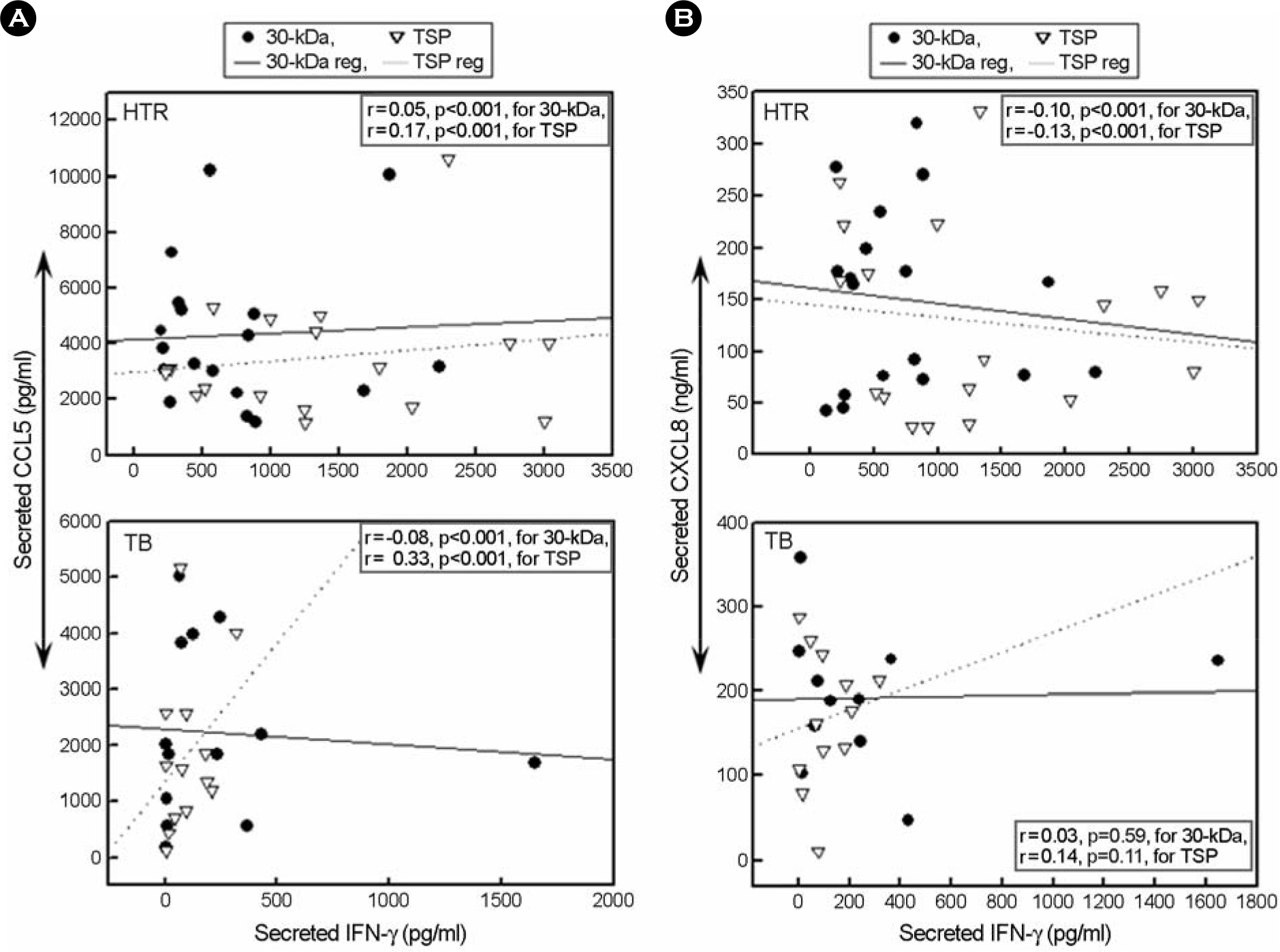
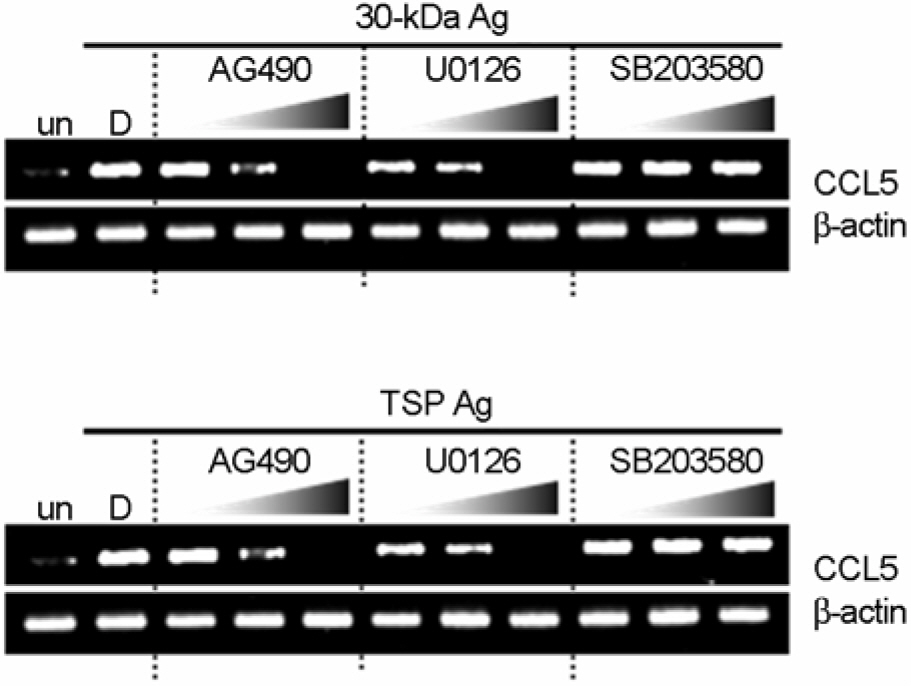
- CCL5/regulated on activation, normal T expressed and secreted production (RANTES) is a principal CC chemokine, and can activate macrophages and Th1 lymphocytes, however, little is known about the CCL5 profiles associated with active tuberculosis (TB). In this study, we investigated the production of CCL5 by the peripheral blood mononuclear cells (PBMCs) of patients with active pulmonary TB after stimulation with Triton X-100 soluble proteins (TSP) or the 30-kDa antigen. The profiles of cytokines/chemokines [CXCL8/interleukin (IL)-8, IL-12 p40, and interferon (IFN)-gamma] were also examined by PBMCs from TB patients, and compared with those obtained from healthy tuberculin reactors (HTR). Concordant with earlier studies, IFN-gamma production was significantly depressed in the PBMCs from TB patients compared with those from HTR. In addition, the CCL5, but not CXCL8, levels in the PBMCs from TB patients were significantly depressed after stimulation for 18 hr compared to those in the PBMCs from HTRs. The CCL5 release was not significantly correlated with the release of IFN-gamma in the cells from TB patients and HTRs. Further, inhibitor studies show that the 30-kDa- or TSP-induced CCL5 mRNA expression is sensitive to inhibitors of mitogen-activated protein kinase kinase (MEK) 1/2 and Janus kinase (JAK) 2, but not p38, pathway activation, suggesting a MEK1/2- or JAK2-based mechanism is responsible for modulating of the CCL5 expression in human PBMCs. Collectively, these data suggest that TB patients show depressed production of CCL5 secretion, which can be modulated by MEK- and JAK2-based transcriptional regulatory mechanisms, in response to the mycobacterial antigens.
Keyword
MeSH Terms
-
Corynebacterium
Humans
Interferon-gamma
Interferons
Interleukin-12
Lymphocytes
Macrophages
Octoxynol
Phosphotransferases
Protein Kinases
Proteins
RNA, Messenger
Tuberculin
Tuberculosis
Tuberculosis, Pulmonary
Corynebacterium
Interferon-gamma
Interferons
Interleukin-12
Octoxynol
Phosphotransferases
Protein Kinases
Proteins
RNA, Messenger
Tuberculin
Figure
Reference
-
1). Aung H., Toossi Z., Wisnieski JJ., Wallis RS., Culp LA., Phillips NB., Phillips M., Averill LE., Daniel TM., Ellner JJ. Induction of monocyte expression of tumor necrosis factor alpha by the 30-kD alpha antigen of Mycobacterium tuberculosis and synergism with fibronectin. J Clin Invest. 98:1261–1268. 1996.2). Bacon KB., Premack BA., Gardner P., Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 269:1727–1730. 1995.
Article3). Campbell EM., Proudfoot AE., Yoshimura T., Allet B., Wells TN., White AM., Westwick J., Watson ML. Recombinant guinea pig and human RANTES activate macrophages but not eosinophils in the guinea pig. J Immunol. 159:1482–1489. 1997.4). Casola A., Henderson A., Liu T., Garofalo RP., Brasier AR. Regulation of RANTES promoter activation in alveolar epithelial cells after cytokine stimulation. Am J Physiol Lung Cell Mol Physiol. 283:L1280–1290. 2002.
Article5). Chensue SW., Warmington KS., Ruth JH., Sanghi PS., Lincoln P., Kunkel SL. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 157:4602–4608. 1996.6). Chensue SW., Warmington K., Ruth JH., Lukacs N., Kunkel SL. Mycobacterial and schistosomal antigenelicited granuloma formation in IFN-gamma and IL-4 knockout mice: analysis of local and regional cytokine and chemokine networks. J Immunol. 159:3565–3573. 1997.7). Chensue SW., Warmington KS., Allenspach EJ., Lu B., Gerard C., Kunkel SL., Lukacs NW. Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol. 163:165–173. 1999.8). Darnell JE Jr., Kerr IM., Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 264:1415–1421. 1994.
Article9). Devergne O., Marfaing-Koka A., Schall TJ., Leger-Ravet MB., Sadick M., Peuchmaur M., Crevon MC., Kim KJ., Schall TT., Kim T., Galanaud P., Emilie D. Production of the RANTES chemokine in delayed-type hypersensitivity reactions: involvement of macrophages and endothelial cells. J Exp Med. 179:1689–1694. 1994.
Article10). Dorner BG., Scheffold A., Rolph MS., Huser MB., Kaufmann SH., Radbruch A., Flesch IE., Kroczek RA. MIP-1alpha, MIP-1beta, RANTES, and ATAC/lymphotactin function together with IFN-gamma as type 1 cytokines. Proc Natl Acad Sci U S A. 99:6181–6186. 2002.11). Fine JS., Rojas-Triana A., Jackson JV., Engstrom LW., Deno GS., Lundell DJ., Bober LA. Impairment of leukocyte trafficking in a murine pleuritis model by IL-4 and IL-10. Inflammation. 27:161–174. 2003.12). Flynn JL., Chan J. Immunology of tuberculosis. Annu Rev Immunol. 19:93–129. 2001.
Article13). Friedland JS., Hartley JC., Hartley CG., Shattock RJ., Griffin GE. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin Exp Immunol. 100:233–238. 1995.14). Hussain S., Zwilling BS., Lafuse WP. Mycobacterium avium infection of mouse macrophages inhibits IFN-gamma Janus kinase-STAT signaling and gene induction by down-regulation of the IFN-gamma receptor. J Immunol. 163:2041–2048. 1999.15). Ihle JN. Cytokine receptor signaling. Nature. 377:591–594. 1995.16). Imai K., Kurita-Ochiai T., Ochiai K. Mycobacterium bovis bacillus Calmette-Guérin infection promotes SOCS induction and inhibits IFN-gamma-stimulated JAK/STAT signaling in J774 macrophages. FEMS Immunol Med Microbiol. 39:173–180. 2003.17). Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 21:279–286. 2008.
Article18). Jo EK., Park JK., Dockrell HM. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr Opin Infect Dis. 16:205–210. 2003.
Article19). Jo EK., Yang CS., Choi CH., Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 9:1087–1098. 2007.
Article20). Kim HJ., Jo EK., Park JK., Lim JH., Min D., Paik TH. Isolation and partial characterisation of the Triton X-100 solubilised protein antigen from Mycobacterium tuberculosis. J Med Microbiol. 48:585–591. 1999.21). Kurashima K., Mukaida N., Fujimura M., Yasui M., Nakazumi Y., Matsuda T., Matsushima K. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 155:1474–1477. 1997.
Article22). Larsen CG., Anderson AO., Oppenheim JJ., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 68:31–36. 1989.23). Larsen CG., Thomsen MK., Gesser B., Thomsen PD., Deleuran BW., Nowak J., Sk⊘dt V., Thomsen HK., Deleuran M., Thestrup-Pedersen K., Harada A., Matsushima K., Menne T. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J Immunol. 155:2151–2157. 1995.24). Lee JS., Song CH., Lim JH., Kim HJ., Park JK., Paik TH., Kim CH., Kong SJ., Shon MH., Jung SS., Jo EK. The production of tumour necrosis factor-alpha is decreased in peripheral blood mononuclear cells from multidrug-resistant tuberculosis patients following stimulation with the 30-kDa antigen of Mycobacterium tuberculosis. Clin Exp Immunol. 132:443–449. 2003.25). Lee JS., Son JW., Jung SB., Kwon YM., Yang CS., Oh JH., Song CH., Kim HJ., Park JK., Paik TH., Jo EK. Ex vivo responses for interferon-gamma and proinflammatory cytokine secretion to low-molecular-weight antigen MTB12 of Mycobacterium tuberculosis during human tuberculosis. Scand J Immunol. 64:145–154. 2006.26). Lee JS., Lee JY., Son JW., Oh JH., Shin DM., Yuk JM., Song CH., Paik TH., Jo EK. Expression and regulation of the CC-chemokine ligand 20 during human tuberculosis. Scand J Immunol. 67:77–85. 2008.
Article27). Lin Y., Gong J., Zhang M., Xue W., Barnes PF. Production of monocyte chemoattractant protein 1 in tuberculosis patients. Infect Immun. 66:2319–2322. 1998.
Article28). Marfaing-Koka A., Devergne O., Gorgone G., Portier A., Schall TJ., Galanaud P., Emilie D. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J Immunol. 154:1870–1878. 1995.29). Ottenhoff TH., Verreck FA., Hoeve MA., van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis (Edinb). 85:53–64. 2005.
Article30). Pan ZZ., Parkyn L., Ray A., Ray P. Inducible lung-specific expression of RANTES: preferential recruitment of neutrophils. Am J Physiol Lung Cell Mol Physiol. 279:L658–666. 2000.
Article31). Peters W., Ernst JD. Mechanisms of cell recruitment in the immune response to Mycobacterium tuberculosis. Microbes Infect. 5:151–158. 2003.32). Reiling N., Blumenthal A., Flad HD., Ernst M., Ehlers S. Mycobacteria-induced TNF-alpha and IL-10 formation by human macrophages is differentially regulated at the level of mitogen-activated protein kinase activity. J Immunol. 167:3339–3345. 2001.33). Roach SK., Schorey JS. Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria. Infect Immun. 70:3040–3052. 2002.
Article34). Sadek MI., Sada E., Toossi Z., Schwander SK., Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 19:513–521. 1998.
Article35). Saukkonen JJ., Bazydlo B., Thomas M., Strieter RM., Keane J., Kornfeld H. Beta-chemokines are induced by Mycobacterium tuberculosis and inhibit its growth. Infect Immun. 70:1684–1693. 2002.36). Skwor TA., Sedberry Allen S., Mackie JT., Russell K., Berghman LR., McMurray DN. BCG vaccination of guinea pigs modulates Mycobacterium tuberculosis-induced CCL5 (RANTES) production in vitro and in vivo. Tuberculosis (Edinb). 86:419–429. 2006.37). Song CH., Kim HJ., Park JK., Lim JH., Kim UO., Kim JS., Paik TH., Kim KJ., Suhr JW., Jo EK. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect Immun. 68:4477–4484. 2000.
Article38). Song CH., Lee JS., Lee SH., Lim K., Kim HJ., Park JK., Paik TH., Jo EK. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 23:194–201. 2003.39). Stellato C., Beck LA., Gorgone GA., Proud D., Schall TJ., Ono SJ., Lichtenstein LM., Schleimer RP. Expression of the chemokine RANTES by a human bronchial epithelial cell line. Modulation by cytokines and glucocorticoids. J Immunol. 155:410–418. 1995.40). Torres M., Herrera T., Villareal H., Rich EA., Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 66:176–180. 1998.41). Wilkinson PC., Newman I. Identification of IL-8 as a locomotor attractant for activated human lymphocytes in mononuclear cell cultures with anti-CD3 or purified protein derivative of Mycobacterium tuberculosis. J Immunol. 149:2689–2694. 1992.42). Wong MM., Fish EN. Chemokines: attractive mediators of the immune response. Semin Immunol. 15:5–14. 2003.
Article43). Yadav M., Roach SK., Schorey JS. Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol. 172:5588–5597. 2004.44). Yang CS., Lee JS., Jung SB., Oh JH., Song CH., Kim HJ., Park JK., Paik TH., Jo EK. Differential regulation of interleukin-12 and tumour necrosis factor-alpha by phosphatidylinositol 3-kinase and ERK 1/2 pathways during Mycobacterium tuberculosis infection. Clin Exp Immunol. 143:150–160. 2006.45). Zhang Y., Broser M., Cohen H., Bodkin M., Law K., Reibman J., Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 95:586–592. 1995.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Cell-Mediated Immunity in Treatment Failure Pulmonary Tuberculosis
- Downregulation of Angiotensin II-Induced 12-Lipoxygenase Expression and Cell Proliferation in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats by CCL5
- A Case of Tuberculosis Verrucosa Cutis with Ulcer in a Patient with Pulmonary Tuberculosis
- Natural Killer Cell Mediated Cytotoxicity in Tuberculosis Patients
- Tuberculosis Verrucosa Cutis in a Patient with Pulmonary Tuberculosis