Korean J Physiol Pharmacol.
2009 Oct;13(5):385-392. 10.4196/kjpp.2009.13.5.385.
Downregulation of Angiotensin II-Induced 12-Lipoxygenase Expression and Cell Proliferation in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats by CCL5
- Affiliations
-
- 1Department of Microbiology, and Aging-associated Vascular Disease Research Center, College of Medicine, Yeungnam University, Daegu 705-717, Korea. heesun@med.yu.ac.kr
- KMID: 1982573
- DOI: http://doi.org/10.4196/kjpp.2009.13.5.385
Abstract
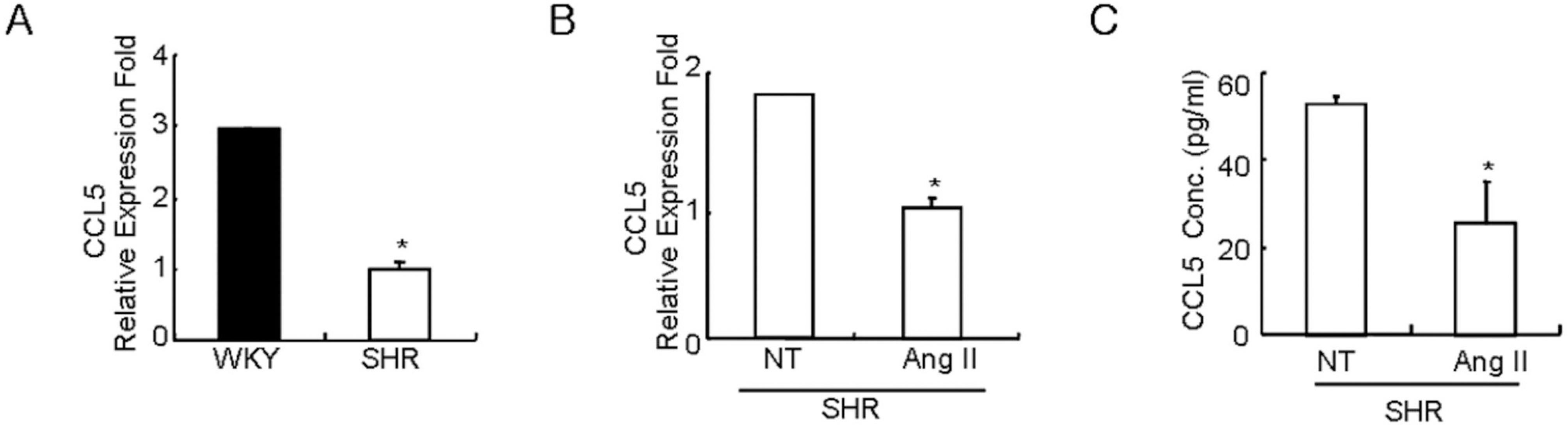
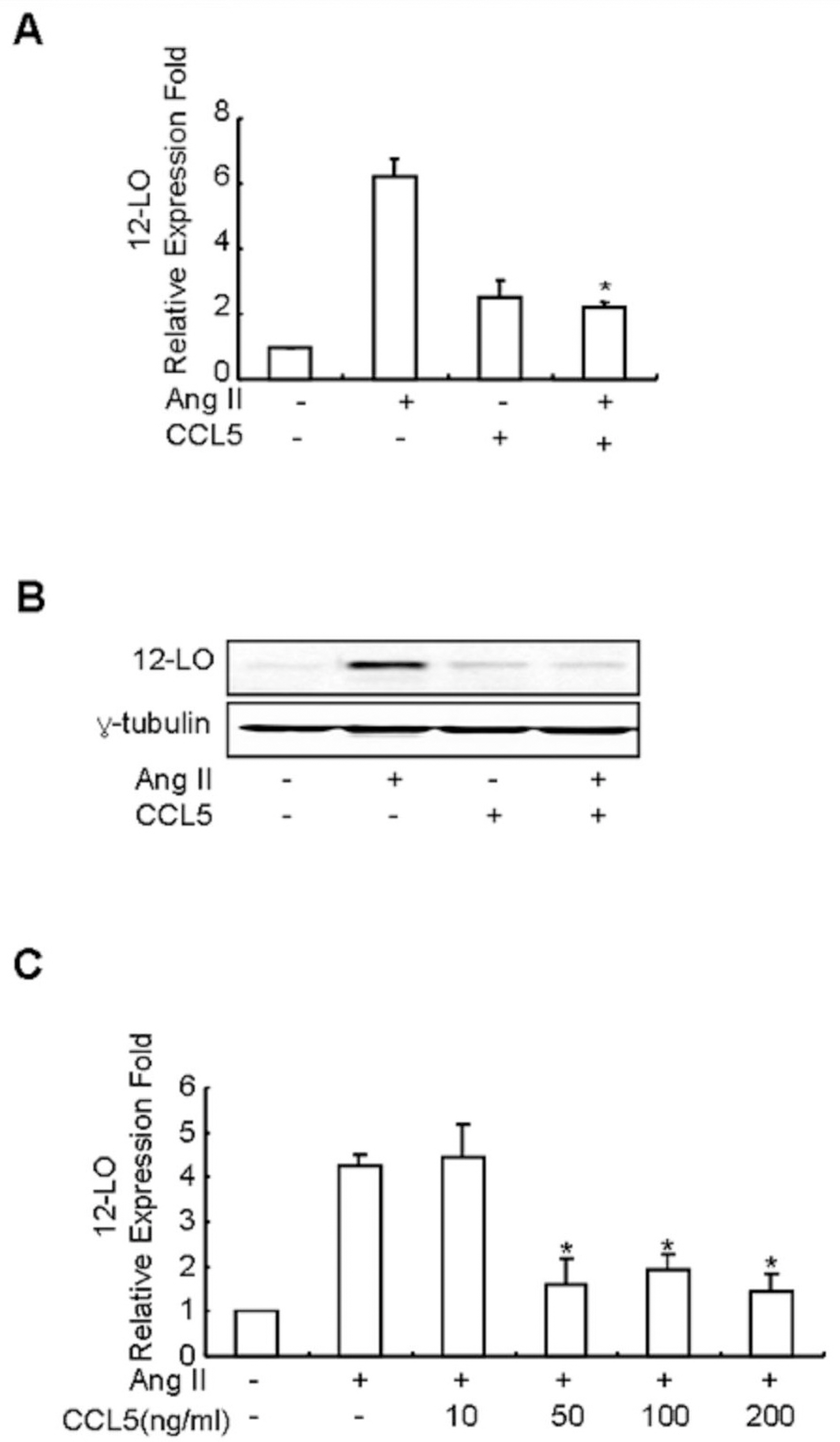
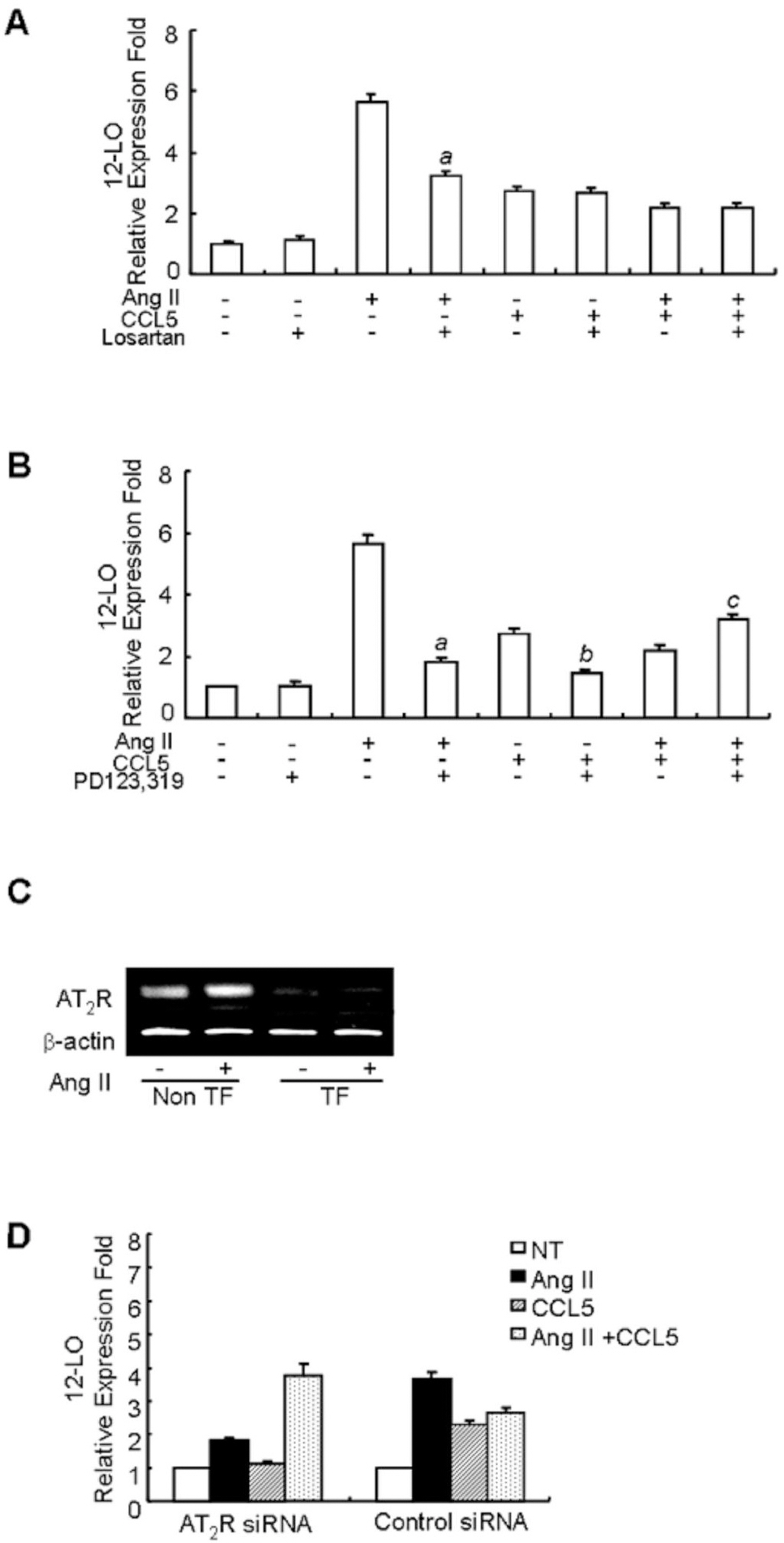
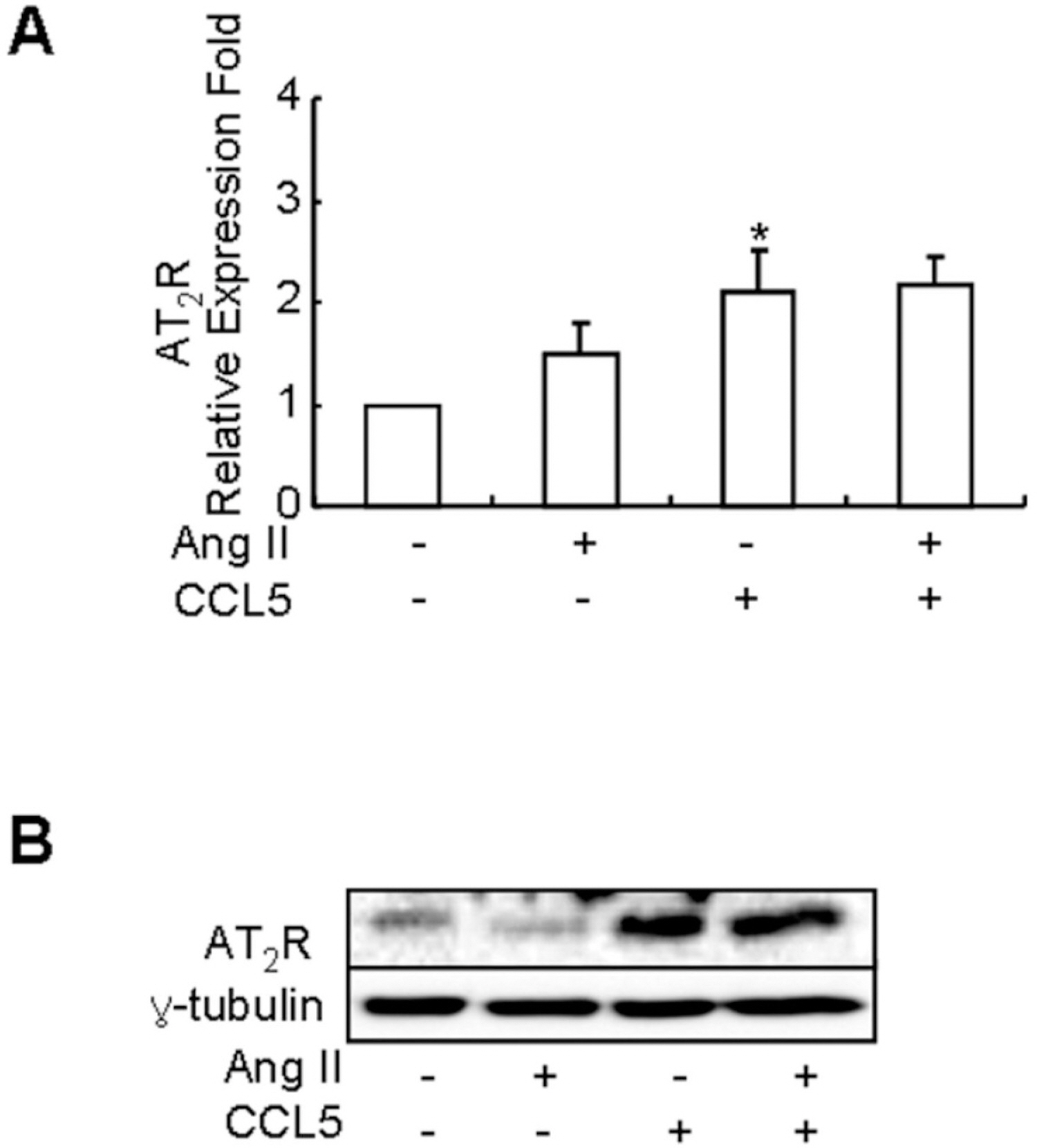
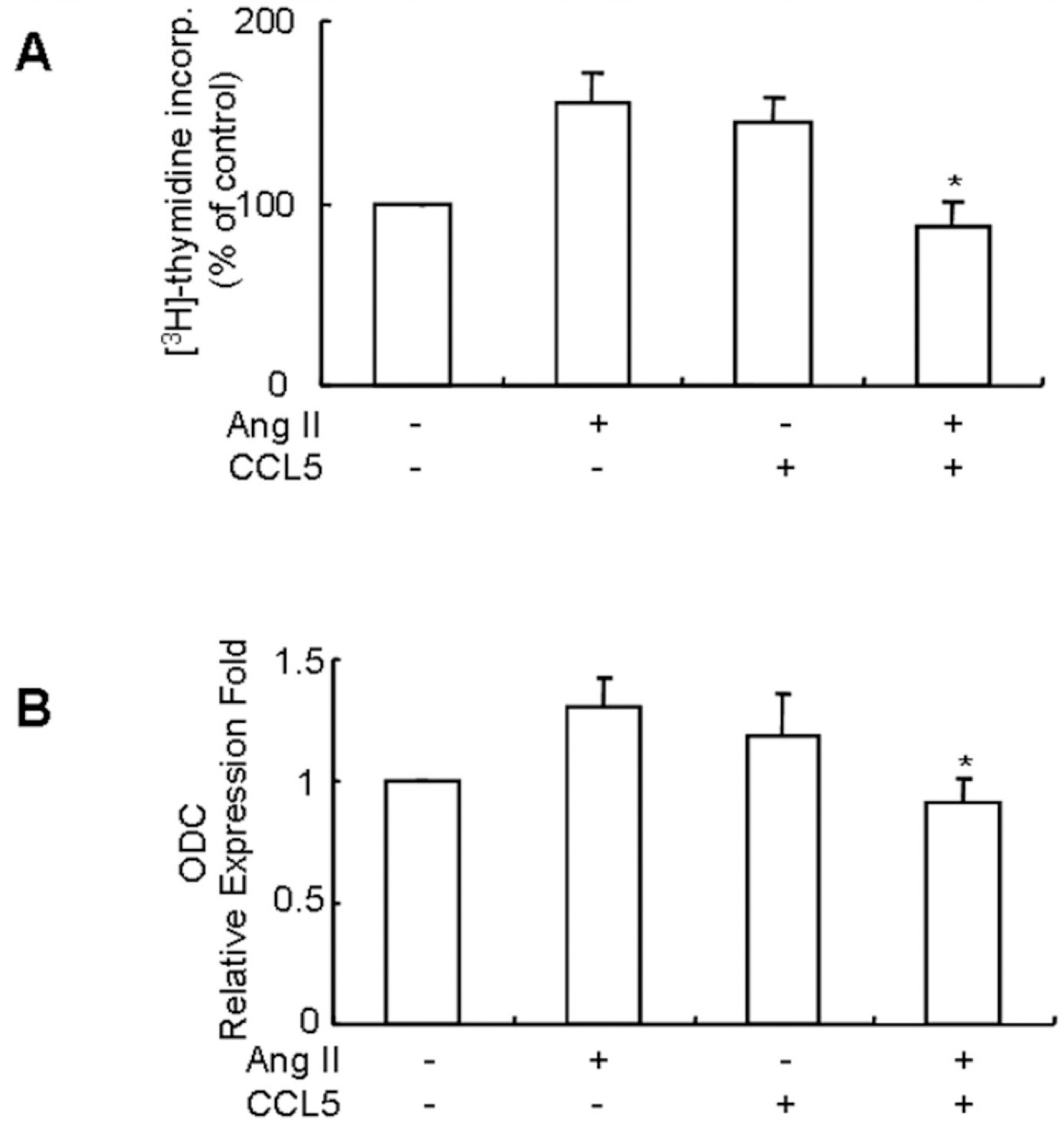
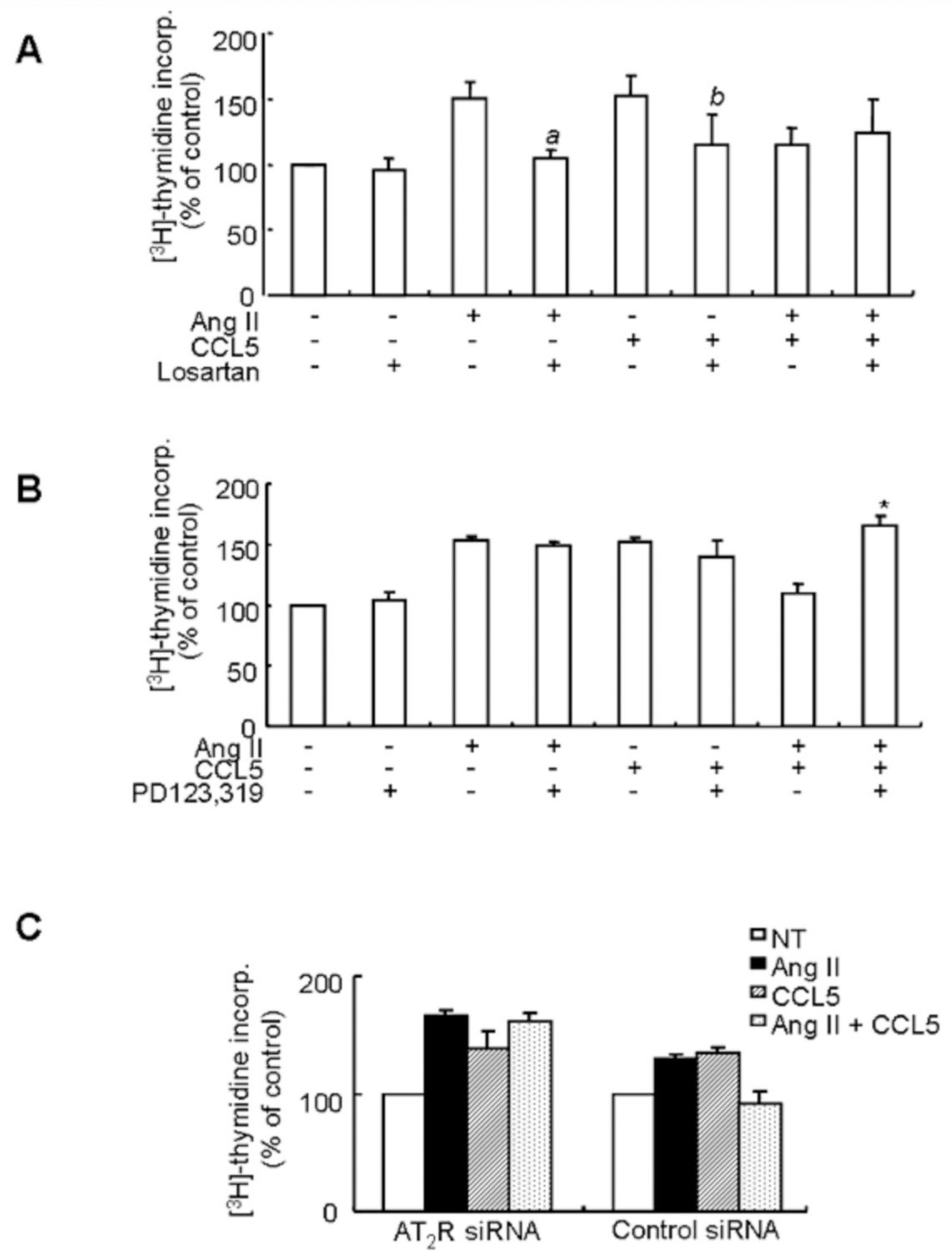
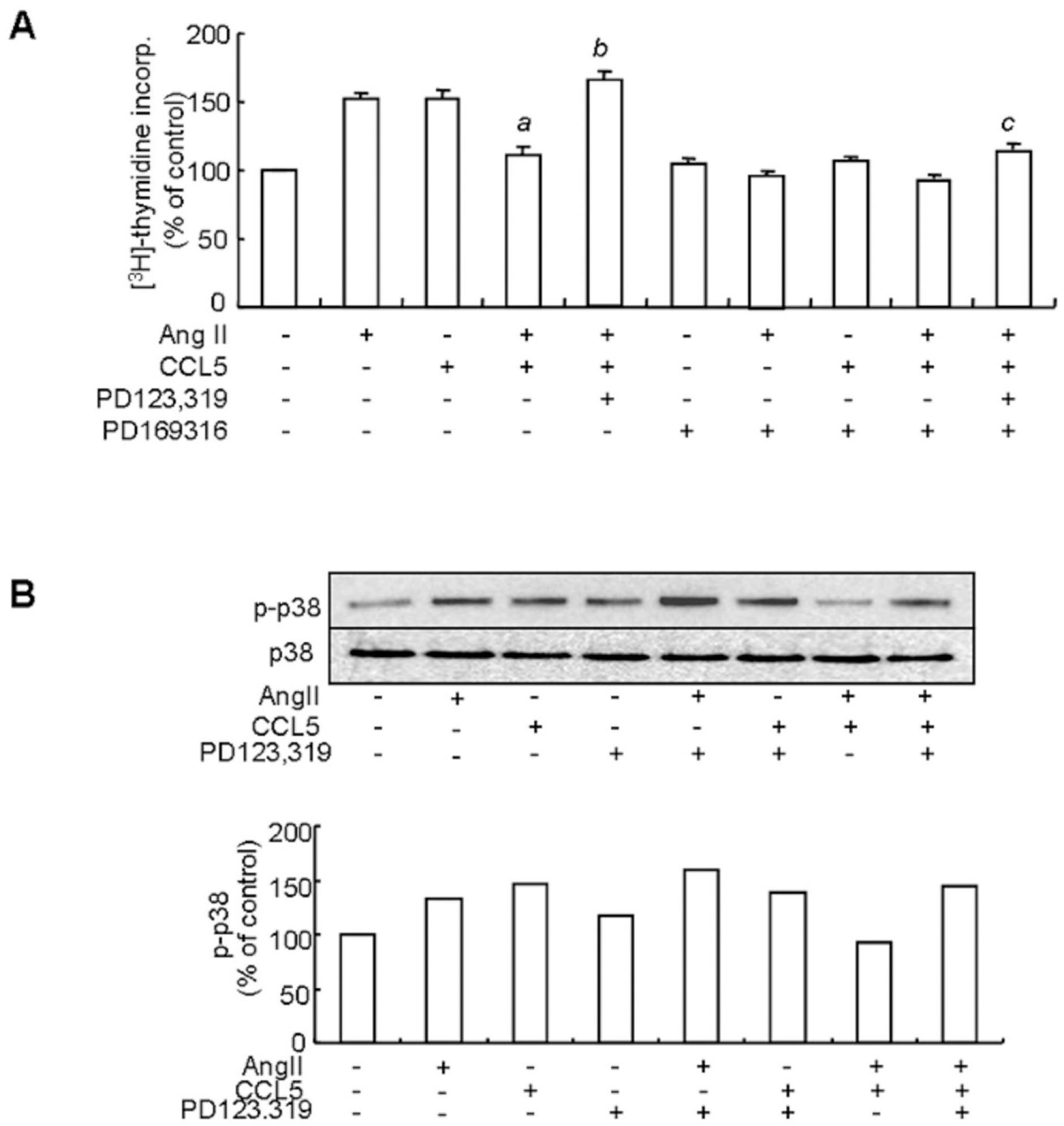
- Angiotensin II (Ang II) plays an important role in vascular hypertension. The role of the chemokine CCL5 on Ang II-induced activities in vascular smooth muscle cells (VSMCs) has not been studied. In this study, we elucidated the effect of CCL5 on Ang II-induced 12-lipoxygenase (LO) expression and cell proliferation in spontaneously hypertensive rats (SHR) VSMCs. CCL5 decreased Ang II-induced 12-LO mRNA expression and protein production, and it increased Ang II type 2 (AT2) receptor expression in SHR VSMCs. The inhibitory effect of CCL5 on Ang II-induced 12-LO mRNA expression was mediated through the AT2 receptor. Although treatment of CCL5 alone induced SHR VSMCs proliferation, CCL5 inhibited Ang II-induced VSMCs proliferation and PD123,319, an AT2 receptor antagonist, blocked the inhibitory effect of CCL5 on Ang II-induced VSMCs proliferation. Phosphorylation of p38 was detected in VSMCs treated with Ang II or CCL5 alone. But, decrease of p38 phosphorylation was detected in VSMCs treated with Ang II and CCL5 simultaneously (Ang II/CCL5) and PD123,319 increased p38 phosphorylation in VSMCs treated with Ang II/CCL5. Therefore, these results suggest that the inhibitory effect of CCL5 on Ang II-induced VSMCs proliferation is mediated by the AT2 receptor via p38 inactivation, and CCL5 may play a beneficial role in Ang II-induced vascular hypertension.
Keyword
MeSH Terms
-
Angiotensin II
Angiotensins
Arachidonate 12-Lipoxygenase
Cell Proliferation
Chemokine CCL5
Down-Regulation
Hypertension
Muscle, Smooth, Vascular
Phosphorylation
Rats, Inbred SHR
Receptor, Angiotensin, Type 2
RNA, Messenger
Angiotensin II
Angiotensins
Arachidonate 12-Lipoxygenase
Chemokine CCL5
RNA, Messenger
Receptor, Angiotensin, Type 2
Figure
Reference
-
References
Bedecs K., Elbaz N., Sutren M., Masson M., Susini C., Strosberg AD. Angiotensin II type 2 receptors mediate inhibition of mitogen activated protein kinase cascade and functional activation of SHP-1 tyrosine phosphatase. Biochem J. 325:449–454. 1997.Buemi M., Marino D., Floccari F., Ruello A., Nostro L., Aloisi C. Effect of interleukin 8 and ICAM-1 on calcium-dependent outflow of K+ in erythrocytes from subjects with essential hypertension. Curr Med Res Opin. 20:19–24. 2004.Chen XL., Tummala PE., Olbrych MT., Alexander RW., Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 83:952–959. 1998.
ArticleDorfm?ller P., Zarka V., Durand-Gasselin I., Monti G., Balabanian K., Garcia G. Chemokine RANES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 165:534–539. 2002.Gallinat S., Busche S., Raizada MK., Sumners C. The angiotesin II type 2 receptor: An enigma with multiple variations. Am J Physiol. 278:E357–E374. 2000.González-Núnez D., Claria J., Rivera F., Roch E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension. 37:334–338. 2001.
ArticleHan Y., Runge MS., Brasier AR. Angiotensin II induced interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kB transcription factor. Circ Res. 84:695–703. 1999.Horiuchi M., Akishita M., Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 33:613–621. 1999.
ArticleHoriuchi M., Lehtonen JY., Daviet L. Signaling mechanism of the AT2 angiotensin II receptor: crosstalk between AT1 and AT2 receptors in cell growth. Trends Endocrinol Metab. 10:391–396. 1999.Ishibashi M., Hiasa KI., Zhao Q., Inoue S., Ohtani K., Kitamoto S. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocyte in hypertension-induced vascular inflammation and remodeling. Circ Res. 94:1203–1210. 2004.Jordan NJ., Watson ml., Williams RJ., Roach AG., Yoshimura T., Westwick J. Chemokine production by human vascular smooth muscle cells: modulation by IL-13. Br J Pharmacol. 122:749–757. 1997.
ArticleKashiwagi M., Masutani K., Shinozaki M., Hirakata H. MCP-1 and RANTES are expressed in renal cortex of rats chronically treated with nitric oxide synthase inhibitor. Nephron. 92:165–173. 2002.
ArticleKim HY., Kang YJ., Song IH., Choi HC., Kim HS. Upregulation of interleukin-8/CXCL8 in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertens Res. 31:515–523. 2008.
ArticleKim JA., Gu JL., Natarajan R., Rerliner JA., Nadler JL. A leukocyte type 12-lipoxygenase is expressed in human vascular and mononuclear cells. Evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Bio. 15:942–948. 1995.Lee HM., Lee CK., Lee SH., Roh HY., Bae YM., Lee KY. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci. 105:74–81. 2007.
ArticleMabrouk ME., Touyz RM., Schiffrin EL. Differential Ang II-induced growth activation pathways in mesenteric artery smooth muscle cells from SHR. Am J Physiol Heart Circ Physiol. 281:H30–H39. 2001.Natarajan R., Gi JL., Rossi J., Gonzales N., Lanting L., Xu L. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci USA. 90:4947–4951. 1993.
ArticleNatarajan R., Rosdahl J., Gonzales N., Bai W. Regulation of 12-lipoxygenase by cytokine in vascular smooth muscle cells. Hypertension. 30:873–879. 1997.Preston IR., Hill NS., Warburton RR., Fanburg BL. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Lung Cell Mol Physiol. 290:L367–L374. 2006.
ArticleResink TJ., Scott-Burden T., Burgin M., Buhler FR. Enhanced responsiveness to angiotensin II in vascular smooth muscle cells from spontaneously hypertensive rats is not associated with alterations in protein kinase C. Hypertension. 14:293–303. 1989.
ArticleRouiz-Ortega M., Lorenzo O., Ruperez M., Konig S., Wittig B., Egido J. Angiotensin II activates nuclear transcription factor Kappa B through At1 and At2 in vascular smooth muscle cells: molecular mechanisms. Circ Res. 86:1266–1272. 2000.Sasaki M., Hori MT., Hino T., Golub MS. Tuck ml. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens. 10:371–378. 1997.Saward L., Zahradka P. The angiotensin type 2 receptor mediates RNA synthesis in A10 vascular smooth muscle cells. J Mol Cell Cardiol. 28:499–506. 1996.
ArticleSchall TJ., Bacon K., Toy KJ., Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 347:669–671. 1990.
ArticleShahrara S., Park CC., Temkin V., Jarvis JW., Volin MV., Pope RM. RANTES modulates TLR4-induced cytokine secretion in human peripheral blood monocytes. J Immunol. 177:5077–5087. 2006.
ArticleTouyz RM., Mabrouk ME., He G., Wu X-H., Schiffrin EL. Mitogen-activated protein/extracellular signal-regulated kinase inhibition attenuates angiotensin II-mediated signaling and contraction in spontaneously hypertensive rat vascular smooth muscle cells. Circ Res. 84:505–515. 1999.
ArticleTripathy D., Thirumangalakudi L., Grammas P. RANTES upregulation in the Alzheimer's disease brain: a possible neuroprotective role. Neurobiol Aging. doi:10.1016/j.neurobiolaging2008.03.009.
ArticleViedt C., Soto U., Krieger-brauer HI., Fei J., Elsing C., Kübler W. Differential activation of mitogen-activated protein kinase in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol. 20:940–948. 2000.Wei LH., Yang Y., Wu G., Ignarro LJ. IL-4 and IL-13 upregulate ornithine decarboxylase expression by PI3K and MAP kinase pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol. 294:C1198–C1205. 2008.
ArticleWilkie N., Ng LL., Boarder MR. Angiotensin II responses of vascular smooth muscle cells from hypertensive rats: enhancedment at the level of p42 and p44 mitogen activated protein kinase. Br J Pharmacol. 122:209–216. 1997.Wolf G., Neilson EG. From converting enzyme inhibition to angiotensin II receptor blockade: New insight on angiotensin II receptor subtypes in the kidney. Exp Nephrol. 4:8–19. 1996.Wolf G., Wenzel U., Burns KD., Harris RC., Stahl RA., Thaiss F. Angiotensin II activates nuclear transcription factor-kB through AT1 and AT2 receptors. Kidney Int. 61:1986–1995. 2002.Wolf G., Ziyadeh FN., Thaiss F., Tomaszewski J., Caron RJ., Wenzel U. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. J Clin Invest. 100:1047–1058. 1997.Zhao M., Liu Y., Bao M., Kato Y., Han J., Eaton JW. Vascular smooth muscle cell proliferation requires both p38 and BMK1 MAP kinases. Arch Biochem Biophys. 400:199–207. 2002.
ArticleZheng Y., Song HJ., Yun SH., Chae YJ., Jia H., Kim CH., Ha TS., Sachinidis A., Ahn HY., Davidge ST. Inhibition of angiotensin ii-induced vascular smooth muscle cell hypertrophy by different catechins. Korean J Physiol Pharmacol. 9:117–123. 2005.Zoja C., Donadelli R., Colleoni S., Figliuzzi M., Bonazzola S., Morigi M. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kB activation. Kidney Int. 53:1608–1615. 1998.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sulfatase 1 mediates the inhibitory effect of angiotensin II type 2 receptor inhibitor on angiotensin II-induced hypertensive mediator expression and proliferation in vascular smooth muscle cells from spontaneously hypertensive rats
- IL-8/CXCL8 Upregulates 12-Lipoxygenase Expression in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats
- CCL5 Inhibits Elevation of Blood Pressure and Expression of Hypertensive Mediators in Developing Hypertension State Spontaneously Hypertensive Rats
- Effect of Age on Proliferation of Rat Vascular Smooth Muscle Cells Stimulated with Fetal Bovine Serum, Angiotensin II and Phorbol 12-Myristate 13-Acetate
- Studies on the structural changes of aortic media and its repairing effect by enalapril in spontaneously hypertensive rats