J Bone Metab.
2020 Feb;27(1):53-63. 10.11005/jbm.2020.27.1.53.
Inhibitory Effect of Rosae Multiflorae Fructus Extracts on the Receptor Activator of NF-κB Ligand-Induced Osteoclastogenesis through Modulation of P38- and Ca2âº-Mediated Nuclear Factor of Activated T-Cells Cytoplasmic 1 Expression
- Affiliations
-
- 1Department of Oral Microbiology and Immunology, College of Dentistry, Wonkwang University, Iksan, Korea. leesh2@wku.ac.kr
- 2Department of Oral Physiology, College of Dentistry, Wonkwang University, Iksan, Korea.
- 3Institute of Biomaterials and Implant, College of Dentistry, Wonkwang University, Iksan, Korea.
- KMID: 2471314
- DOI: http://doi.org/10.11005/jbm.2020.27.1.53
Abstract
- BACKGROUND
Rosae Multiflorae fructus (RMF), known to have anti-inflammatory and antioxidant properties, has been used as a traditional remedy for inflammatory diseases such as arthritis in Eastern Asia. However, its effect on osteoclasts, which play a crucial role in resorptive inflammatory bone diseases, is yet to be elucidated.
METHODS
The effect of extract of RMF (RMF-E) on receptor activator of nuclear factor-κB ligand (RANKL)-mediated osteoclastogenesis was examined by tartrate-resistant acid phosphatase (TRAP) staining, real-time polymerase chain reaction and western blot analysis. In addition, RANKL-induced Ca2âº-oscillation was also investigated.
RESULTS
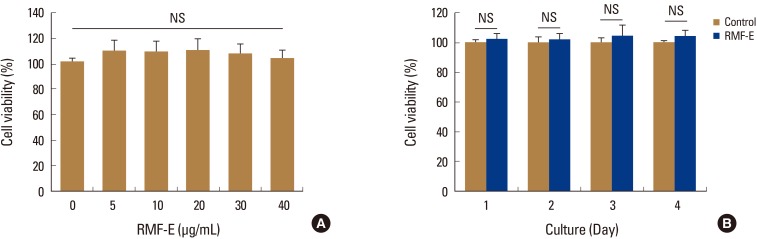
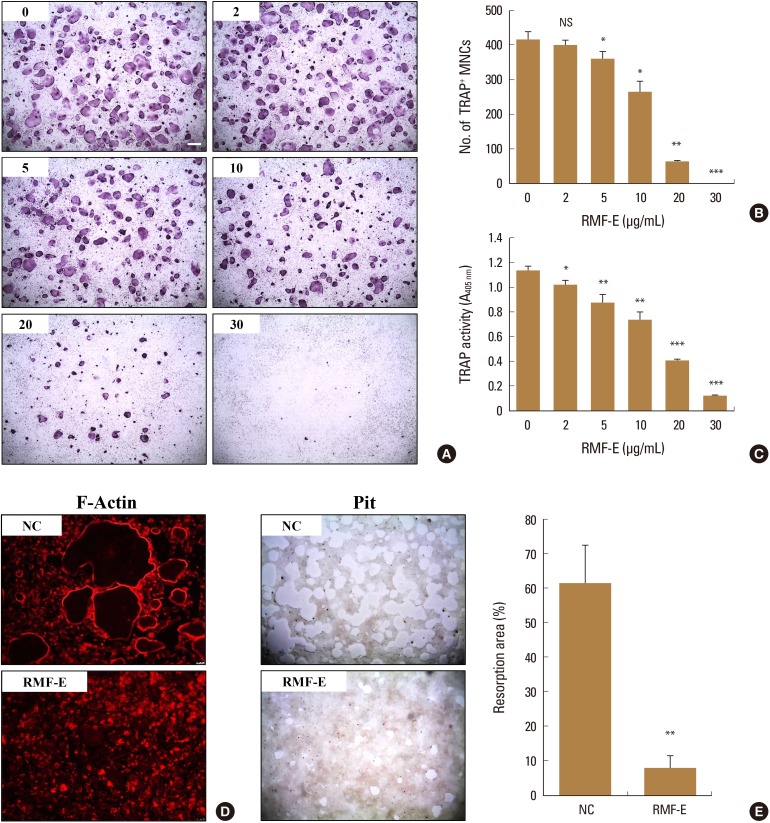
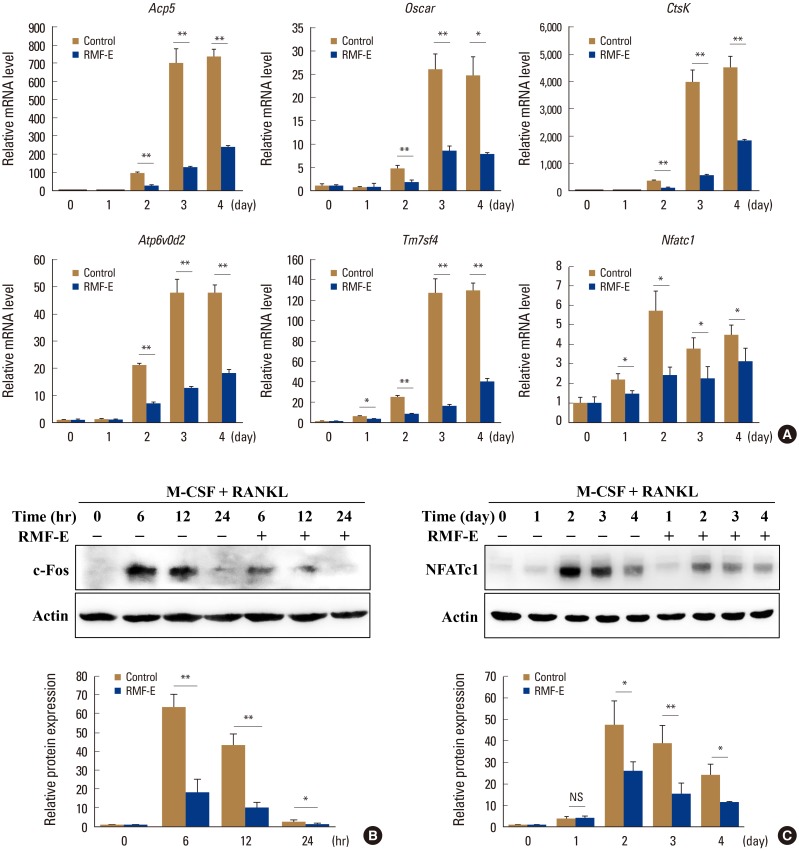
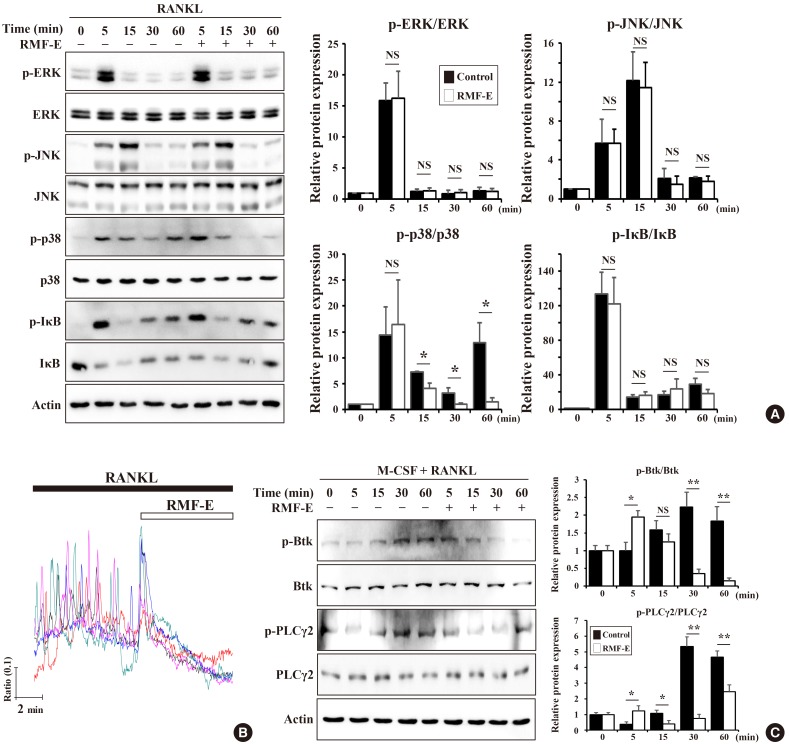
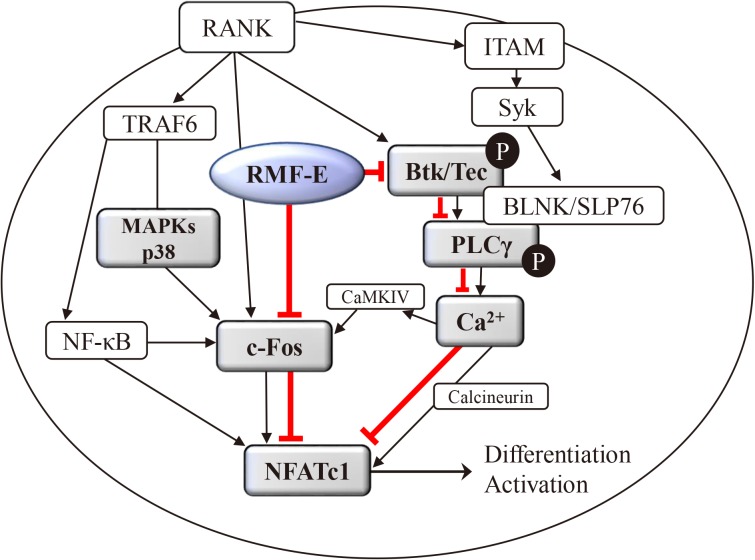
RMF-E remarkably inhibited TRAP+-osteoclast and resorptive pit formation in a dose-dependent manner. In addition, the expression of c-Fos and nuclear factor of activated T-cells cytoplasmic, known as pivotal transcription factors for osteoclast formation in vitro and in vivo, and that of the osteoclast differentiation markers such as Acp5, Oscar, CtsK, Atp6v0d2, Tm7sf4, and Nfatc1 were significantly decreased by RMF-E treatment during osteoclastogenesis. The inhibitory effect of RMF-E on RANKL-induced osteoclastogenesis was caused by the suppression of p38 mitogen-activated protein kinase activation, and RANKL-induced Ca2âº-oscillation removal via inactivation of Bruton's tyrosine kinase (BTK), and subsequently phospholipase C-γ2.
CONCLUSIONS
RMF-E negatively regulates osteoclast differentiation and formation. These findings suggest the possibility of RMF-E as a traditional therapeutic agent against osteoclast-related bone disorders such as osteoporosis, rheumatoid arthritis, and periodontitis.
Keyword
MeSH Terms
-
Acid Phosphatase
Antigens, Differentiation
Arthritis
Arthritis, Rheumatoid
Blotting, Western
Bone Diseases
Calcium Signaling
Cytoplasm*
Far East
In Vitro Techniques
Osteoclasts
Osteogenesis
Osteoporosis
Periodontitis
Phospholipases
Protein Kinases
Protein-Tyrosine Kinases
Real-Time Polymerase Chain Reaction
Rosa*
T-Lymphocytes*
Transcription Factors
Acid Phosphatase
Antigens, Differentiation
Phospholipases
Protein Kinases
Protein-Tyrosine Kinases
Transcription Factors
Figure
Reference
-
1. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342. PMID: 12748652.
Article2. Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006; 24:33–63. PMID: 16551243.
Article3. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014; 5:511. PMID: 25368616.
Article4. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007; 7:292–304. PMID: 17380158.
Article5. Shinohara M, Koga T, Okamoto K, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008; 132:794–806. PMID: 18329366.
Article6. Lee SH, Kim T, Jeong D, et al. The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation. J Biol Chem. 2008; 283:11526–11534. PMID: 18281276.
Article7. Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009; 231:241–256. PMID: 19754901.
Article8. Kim H, Kim T, Jeong BC, et al. Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell Metab. 2013; 17:249–260. PMID: 23395171.
Article9. Kim JH, Kim N. Regulation of NFATc1 in osteoclast differentiation. J Bone Metab. 2014; 21:233–241. PMID: 25489571.
Article10. Cheng BC, Ma XQ, Kwan HY, et al. A herbal formula consisting of Rosae Multiflorae Fructus and Lonicerae Japonicae Flos inhibits inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. J Ethnopharmacol. 2014; 153:922–927. PMID: 24568773.
Article11. Cheng BC, Yu H, Guo H, et al. A herbal formula comprising Rosae Multiflorae Fructus and Lonicerae Japonicae Flos, attenuates collagen-induced arthritis and inhibits TLR4 signalling in rats. Sci Rep. 2016; 6:20042. PMID: 26860973.
Article12. Song CH, Bui TT, Piao CH, et al. Rosae Multiflorae fructus hot water extract inhibits a murine allergic asthma via the suppression of Th2 cytokine production and histamine release from mast cells. J Med Food. 2016; 19:853–859. PMID: 27574849.
Article13. Kitahiro Y, Ikeda H, Im HT, et al. Phytochemical characterization of Rosa multiflora Thunb. (Rosaceae) in Japan and South Korea, with a focus on the bioactive flavonol glycoside ‘multiflorin A’. J Nat Med. 2019; 73:555–565. PMID: 30949951.
Article14. Yi O, Jovel EM, Towers GH, et al. Antioxidant and antimicrobial activities of native Rosa sp. from British Columbia, Canada. Int J Food Sci Nutr. 2007; 58:178–189. PMID: 17514536.15. Sacan O, Yanardag R. Purification and some properties of rose (Fructus cynosbati) hips invertase. Indian J Biochem Biophys. 2012; 49:109–114. PMID: 22650008.16. Chrubasik JE, Roufogalis BD, Chrubasik S. Evidence of effectiveness of herbal antiinflammatory drugs in the treatment of painful osteoarthritis and chronic low back pain. Phytother Res. 2007; 21:675–683. PMID: 17444576.
Article17. Zhang GQ, Huang XD, Wang H, et al. Anti-inflammatory and analgesic effects of the ethanol extract of Rosa multiflora Thunb. hips. J Ethnopharmacol. 2008; 118:290–294. PMID: 18515025.
Article18. Bui TT, Kwon DA, Choi DW, et al. Rosae multiflorae fructus extract and its four active components alleviate ovalbumin-induced allergic inflammatory responses via regulation of Th1/Th2 imbalance in BALB/c rhinitis mice. Phytomedicine. 2019; 55:238–248. PMID: 30668435.
Article19. Cheng BC, Yu H, Su T, et al. A herbal formula comprising Rosae Multiflorae Fructus and Lonicerae Japonicae Flos inhibits the production of inflammatory mediators and the IRAK-1/TAK1 and TBK1/IRF3 pathways in RAW 264.7 and THP-1 cells. J Ethnopharmacol. 2015; 174:195–199. PMID: 26297845.
Article20. Park KH, Gu DR, So HS, et al. Dual role of cyanidin-3-glucoside on the differentiation of bone cells. J Dent Res. 2015; 94:1676–1683. PMID: 26350961.
Article21. Jin SH, Kim H, Gu DR, et al. Actin-binding LIM protein 1 regulates receptor activator of NF-kappaB ligand-mediated osteoclast differentiation and motility. BMB Rep. 2018; 51:356–361. PMID: 29921413.22. Gu DR, Hwang JK, Erkhembaatar M, et al. Inhibitory effect of chrysanthemum zawadskii herbich var. latilobum kitamura extract on RANKL-induced osteoclast differentiation. Evid Based Complement Alternat Med. 2013; 2013:509482. PMID: 24174976.23. Jia M, Nie Y, Cao DP, et al. Potential antiosteoporotic agents from plants: a comprehensive review. Evid Based Complement Alternat Med. 2012; 2012:364604. PMID: 23365596.
Article24. Lee J, Kim HH. Methanol extract of croton pycnanthus benth. inhibits osteoclast differentiation by suppressing the MAPK and NF-kappaB signaling pathways. J Bone Metab. 2014; 21:269–275. PMID: 25489576.25. Kim JM, Lee JH, Lee GS, et al. Sophorae Flos extract inhibits RANKL-induced osteoclast differentiation by suppressing the NF-kappaB/NFATc1 pathway in mouse bone marrow cells. BMC Complement Altern Med. 2017; 17:164. PMID: 28335757.
Article26. Ha H, Shim KS, Kim T, et al. Water extract of the fruits of Alpinia oxyphylla inhibits osteoclast differentiation and bone loss. BMC Complement Altern Med. 2014; 14:352. PMID: 25249312.
Article27. Park KH, Gu DR, Jin SH, et al. Pueraria lobate inhibits RANKL-Mediated osteoclastogenesis via downregulation of CREB/PGC1beta/c-Fos/NFATc1 signaling. Am J Chin Med. 2017; 45:1725–1744. PMID: 29121799.28. Rein E, Kharazmi A, Winther K. A herbal remedy, Hyben Vital (stand. powder of a subspecies of Rosa canina fruits), reduces pain and improves general wellbeing in patients with osteoarthritis--a double-blind, placebo-controlled, randomised trial. Phytomedicine. 2004; 11:383–391. PMID: 15330493.
Article29. Warholm O, Skaar S, Hedman E, et al. The effects of a standardized Herbal remedy made from a subtype of rosa canina in patients with osteoarthritis: A double-blind, randomized, placebo-controlled clinical trial. Curr Ther Res Clin Exp. 2003; 64:21–31. PMID: 24944354.
Article30. Hwang JK, Erkhembaatar M, Gu DR, et al. Glechoma hederacea suppresses RANKL-mediated osteoclastogenesis. J Dent Res. 2014; 93:685–690. PMID: 24850617.
Article31. Kawakami S, Matsunami K, Otsuka H, et al. Chemical constituents of imported Rosae fructus. J Nat Med. 2009; 63:46–51. PMID: 18855100.
Article32. Wattel A, Kamel S, Prouillet C, et al. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J Cell Biochem. 2004; 92:285–295. PMID: 15108355.33. Lee WS, Lee EG, Sung MS, et al. Kaempferol inhibits IL-1beta-stimulated, RANKL-mediated osteoclastogenesis via downregulation of MAPKs, c-Fos, and NFATc1. Inflammation. 2014; 37:1221–1230. PMID: 24696323.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Humanin suppresses receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation via AMP-activated protein kinase activation
- Inhibitory effect of Chaenomelis Fructus ethanol extract on receptor activator of nuclear factor-kappa B ligand-mediated osteoclastogenesis
- NF-kappaB-Mediated Regulation of Osteoclastogenesis
- Fexaramine Inhibits Receptor Activator of Nuclear Factor-κB Ligand-induced Osteoclast Formation via Nuclear Factor of Activated T Cells Signaling Pathways
- Adseverin mediates RANKL-induced osteoclastogenesis by regulating NFATc1