Korean J Physiol Pharmacol.
2020 Jan;24(1):39-46. 10.4196/kjpp.2020.24.1.39.
β-Sitosterol treatment attenuates cognitive deficits and prevents amyloid plaque deposition in amyloid protein precursor/presenilin 1 mice
- Affiliations
-
- 1Hebei University of Chinese Medicine, Shijiazhang 050200, Hebei province, China.
- 2Neurobiology Laboratory, Institute of Basic Medicine, Hebei Medical University, Shijiazhang 050017, Hebei province, China. machangsheng0311@163.com
- KMID: 2466568
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.39
Abstract
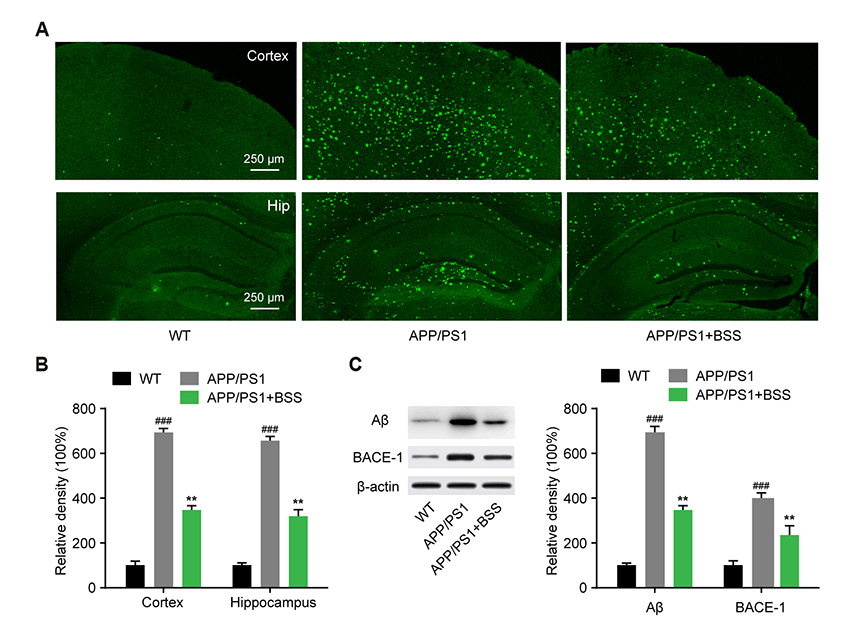
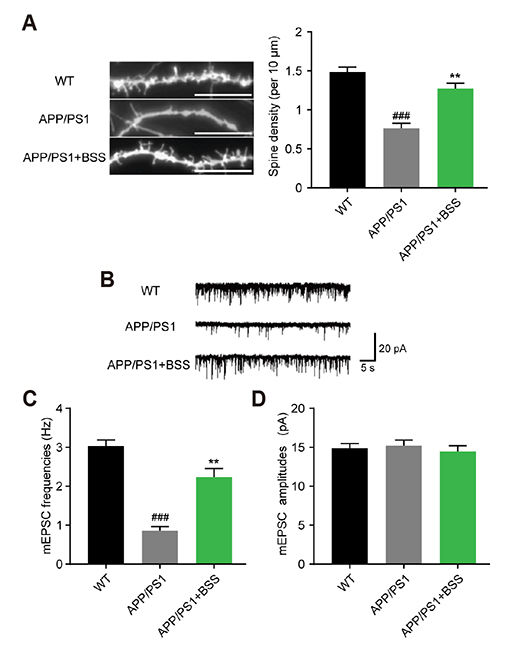
- Alzheimer's disease (AD) is the most common neurodegenerative disorder causing dementia worldwide, and is mainly characterized by aggregated β-amyloid (Aβ). Increasing evidence has shown that plant extracts have the potential to delay AD development. The plant sterol β-Sitosterol has a potential role in inhibiting the production of platelet Aβ, suggesting that it may be useful for AD prevention. In the present study, we aimed to investigate the effect and mechanism of β-Sitosterol on deficits in learning and memory in amyloid protein precursor/presenilin 1 (APP/PS1) double transgenic mice. APP/PS1 mice were treated with β-Sitosterol for four weeks, from the age of seven months. Brain Aβ metabolism was evaluated using ELISA and Western blotting. We found that β-Sitosterol treatment can improve spatial learning and recognition memory ability, and reduce plaque load in APP/PS1 mice. β-Sitosterol treatment helped reverse dendritic spine loss in APP/PS1 mice and reversed the decreased hippocampal neuron miniature excitatory postsynaptic current frequency. Our research helps to explain and support the neuroprotective effect of β-Sitosterol, which may offer a novel pharmaceutical agent for the treatment of AD. Taken together, these findings suggest that β-Sitosterol ameliorates memory and learning impairment in APP/PS1 mice and possibly decreases Aβ deposition.
MeSH Terms
-
Alzheimer Disease
Amyloid*
Animals
Blood Platelets
Blotting, Western
Brain
Cognition Disorders*
Dementia
Dendritic Spines
Enzyme-Linked Immunosorbent Assay
Excitatory Postsynaptic Potentials
Learning
Memory
Metabolism
Mice*
Mice, Transgenic
Neurodegenerative Diseases
Neurons
Neuroprotective Agents
Plant Extracts
Plants
Plaque, Amyloid*
Spatial Learning
Amyloid
Neuroprotective Agents
Plant Extracts
Figure
Reference
-
1. Aprahamian I, Stella F, Forlenza OV. New treatment strategies for Alzheimer's disease: is there a hope? Indian J Med Res. 2013; 138:449–460.2. Zhang LH, Wang X, Stoltenberg M, Danscher G, Huang L, Wang ZY. Abundant expression of zinc transporters in the amyloid plaques of Alzheimer's disease brain. Brain Res Bull. 2008; 77:55–60.
Article3. Ma SL, Pastorino L, Zhou XZ, Lu KP. Prolyl isomerase Pin1 promotes amyloid precursor protein (APP) turnover by inhibiting glycogen synthase kinase-3β (GSK3β) activity: novel mechanism for Pin1 to protect against Alzheimer disease. J Biol Chem. 2012; 287:6969–6973.4. Bateman RJ. Amyloid-beta production and clearance rates in Alzheimer's disease. J Alzheimer's Assoc. 2010; 6:4 Suppl. S101.5. Gordon MN, King DL, Diamond DM, Jantzen PT, Boyett KV, Hope CE, Hatcher JM, DiCarlo G, Gottschall WP, Morgan D, Arendash GW. Correlation between cognitive deficits and Abeta deposits in transgenic APP+PS1 mice. Neurobiol Aging. 2001; 22:377–385.6. Guo LL, Guan ZZ, Huang Y, Wang YL, Shi JS. The neurotoxicity of β-amyloid peptide toward rat brain is associated with enhanced oxidative stress, inflammation and apoptosis, all of which can be attenuated by scutellarin. Exp Toxicol Pathol. 2013; 65:579–584.
Article7. Cynis H, Frost JL, Crehan H, Lemere CA. Immunotherapy targeting pyroglutamate-3 Aβ: prospects and challenges. Mol Neurodegener. 2016; 11:48.
Article8. Tewari D, Stankiewicz AM, Mocan A, Sah AN, Tzvetkov NT, Huminiecki L, Horbańczuk JO, Atanasov AG. Ethnopharmacological approaches for dementia therapy and significance of natural products and herbal drugs. Front Aging Neurosci. 2018; 10:3.
Article9. Liu YX, Huang HC, Chang P, Jiang ZF. Mechanisms of neuroprotective activities of catechins in Alzheimer's disease. Nat Prod Res Dev. 2013; (11):1607–1613.10. Hamaguchi T, Ono K, Yamada M. REVIEW: Curcumin and Alzheimer's disease. CNS Neurosci Ther. 2010; 16:285–297.
Article11. Benesch MG, McElhaney RN. A comparative calorimetric study of the effects of cholesterol and the plant sterols campesterol and brassicasterol on the thermotropic phase behavior of dipalmitoylphosphatidylcholine bilayer membranes. Biochim Biophys Acta. 2014; 1838:1941–1949.
Article12. Baskar AA, Al Numair KS, Gabriel Paulraj M, Alsaif MA, Muamar MA, Ignacimuthu S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J Med Food. 2012; 15:335–343.
Article13. Burg VK, Grimm HS, Rothhaar TL, Grösgen S, Hundsdörfer B, Haupenthal VJ, Zimmer VC, Mett J, Weingärtner O, Laufs U, Broersen LM, Tanila H, Vanmierlo T, Lütjohann D, Hartmann T, Grimm MO. Plant sterols the better cholesterol in Alzheimer's disease? A mechanistical study. J Neurosci. 2013; 33:16072–16087.
Article14. Wang J, Wu F, Shi C. Substitution of membrane cholesterol with β-sitosterol promotes nonamyloidogenic cleavage of endogenous amyloid precursor protein. Neuroscience. 2013; 247:227–233.
Article15. Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006; 24:516–524.
Article16. Sadowski M, Pankiewicz J, Scholtzova H, Ji Y, Quartermain D, Jensen CH, Duff K, Nixon RA, Gruen RJ, Wisniewski T. Amyloid-beta deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice. J Neuropathol Exp Neurol. 2004; 63:418–428.17. Stavnezer AJ, Hyde LA, Bimonte HA, Armstrong CM, Denenberg VH. Differential learning strategies in spatial and nonspatial versions of the Morris water maze in the C57BL/6J inbred mouse strain. Behav Brain Res. 2002; 133:261–270.
Article18. Folci A, Murru L, Vezzoli E, Ponzoni L, Gerosa L, Moretto E, Longo F, Zapata J, Braida D, Pistillo F, Bähler M, Francolini M, Sala M, Bassani S. Myosin IXa binds AMPAR and regulates synaptic structure, LTP, and cognitive function. Front Mol Neurosci. 2016; 9:1.
Article19. Elliott E, Laufer O, Ginzburg I. BAG-1M is up-regulated in hippocampus of Alzheimer's disease patients and associates with tau and APP proteins. J Neurochem. 2009; 109:1168–1178.
Article20. Takata K, Kitamura Y, Taniguchi T. Pathological changes induced by amyloid-β in Alzheimer's disease. Yakugaku Zasshi. 2011; 131:3–11.
Article21. Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008; 28:6333–6341.22. Saeed AA, Genové G, Li T, Hülshorst F, Betsholtz C, Björkhem I, Lütjohann D. Increased flux of the plant sterols campesterol and sitosterol across a disrupted blood brain barrier. Steroids. 2015; 99(Pt B):183–188.
Article23. Shi C, Liu J, Wu F, Zhu X, Yew DT, Xu J. β-sitosterol inhibits high cholesterol-induced platelet β-amyloid release. J Bioenerg Biomembr. 2011; 43:691–697.
Article24. Zhang MY, Zheng CY, Zou MM, Zhu JW, Zhang Y, Wang J, Liu CF, Li QF, Xiao ZC, Li S, Ma QH, Xu RX. Lamotrigine attenuates deficits in synaptic plasticity and accumulation of amyloid plaques in APP/PS1 transgenic mice. Neurobiol Aging. 2014; 35:2713–2725.
Article25. Guénette SY. Mechanisms of Abeta clearance and catabolism. Neuromolecular Med. 2003; 4:147–160.26. Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002; 22:1858–1867.27. Zhang RS, Xu HJ, Jiang JH, Han RW, Chang M, Peng YL, Wang Y, Wang R. Endomorphin-1 attenuates Aβ42 induced impairment of novel object and object location recognition tasks in mice. Brain Res. 2015; 1629:210–220.
Article28. Holtzman DM, Zlokovic B. Role of A β transport and clearance in the pathogenesis and treatment of Alzheimer's disease. In : Sisodia SS, Tanzi RE, editors. Alzheimer's disease. Boston, MA: Springer;2007. p. 179–198.29. Li W, Yu J, Liu Y, Huang X, Abumaria N, Zhu Y, Huang X, Xiong W, Ren C, Liu XG, Chui D, Liu G. Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer's disease mouse model. Mol Brain. 2014; 7:65.
Article30. Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004; 62:925–931.31. Perez-Cruz C, Nolte MW, van Gaalen MM, Rustay NR, Termont A, Tanghe A, Kirchhoff F, Ebert U. Reduced spine density in specific regions of CA1 pyramidal neurons in two transgenic mouse models of Alzheimer's disease. J Neurosci. 2011; 31:3926–3934.
Article32. Duffy FH, Albert MS, McAnulty G. Brain electrical activity in patients with presenile and senile dementia of the Alzheimer type. Ann Neurol. 1984; 16:439–448.
Article