Allergy Asthma Respir Dis.
2019 Jul;7(3):150-157. 10.4168/aard.2019.7.3.150.
Development of Aspergillus fumigatus-induced chronic atopic dermatitis mouse model
- Affiliations
-
- 1Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
- 2Department of Pediatrics, Asan Medical Center, Seoul, Korea. jinhoyu@amc.seoul.kr
- KMID: 2461408
- DOI: http://doi.org/10.4168/aard.2019.7.3.150
Abstract
- PURPOSE
Atopic dermatitis (AD) is the most common chronic and relapsing inflammatory skin disease with skin barrier defects and altered immune responses. Chronic inflammation leads to irreversible fibrosis in the skin and there is no treatment to completely abolish the inflammation and fibrosis. To prevent or treat the chronic process of AD, it is necessary to develop a murine model of AD that reflects the chronic process to identify the mechanism. The aims of this study were to develop a chronic AD model with a crude extract Aspergillus fumigatus (Af) antigen.
METHODS
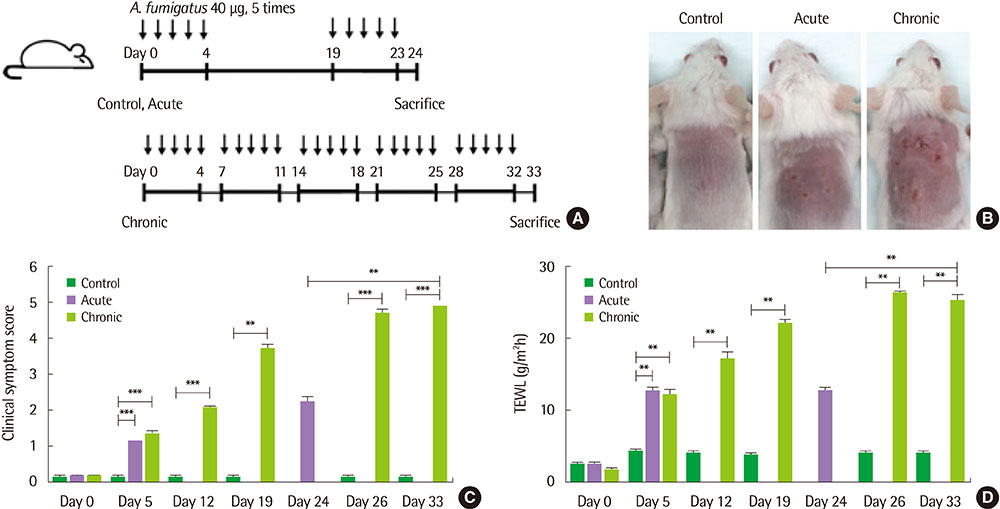
We applied Af extract (40 µg) epicutaneously to the dorsal skin of BALB/c mice for 5 consecutive days per week during a period of 5 weeks for a chronic AD model, and 5 consecutive days repeatedly with 2 weeks interval for an acute AD model.
RESULTS
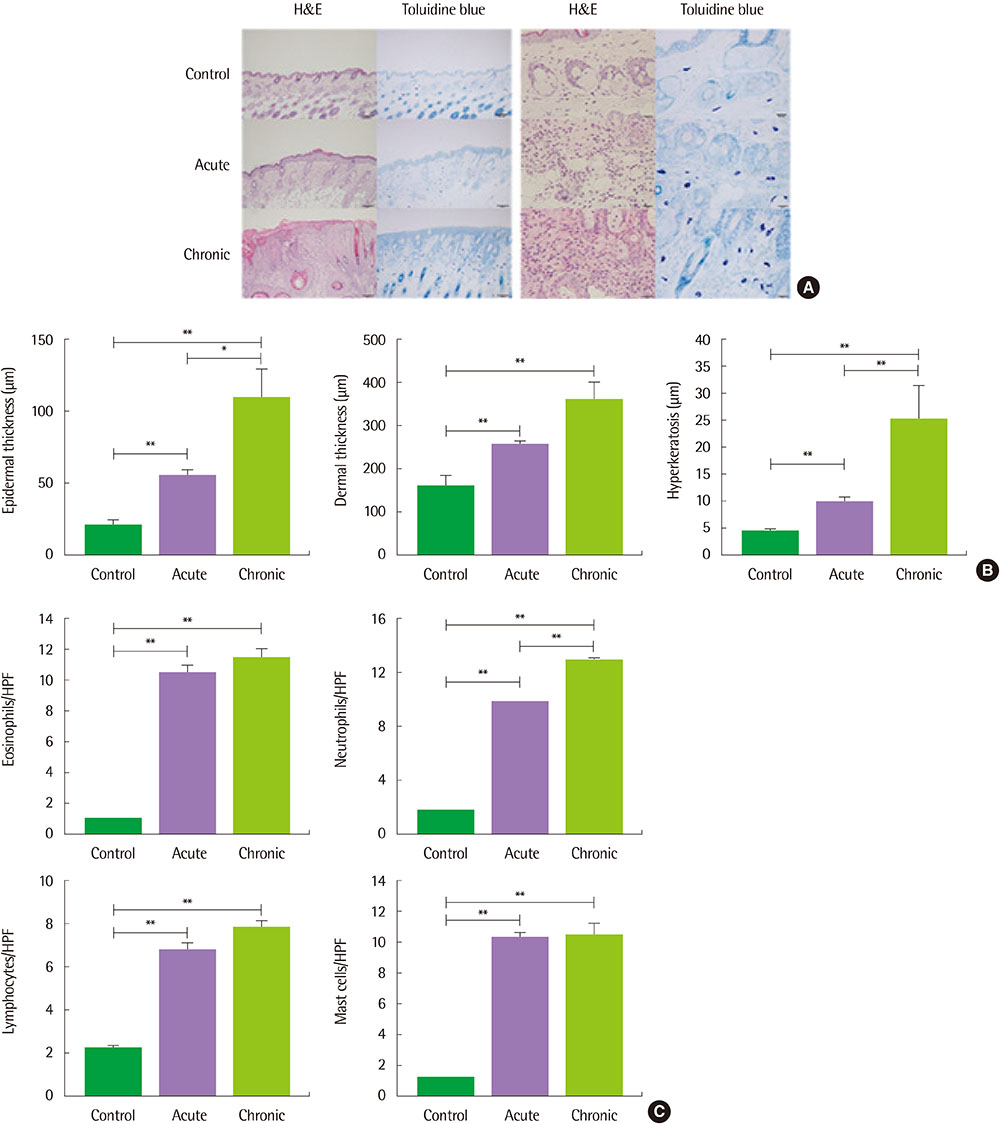
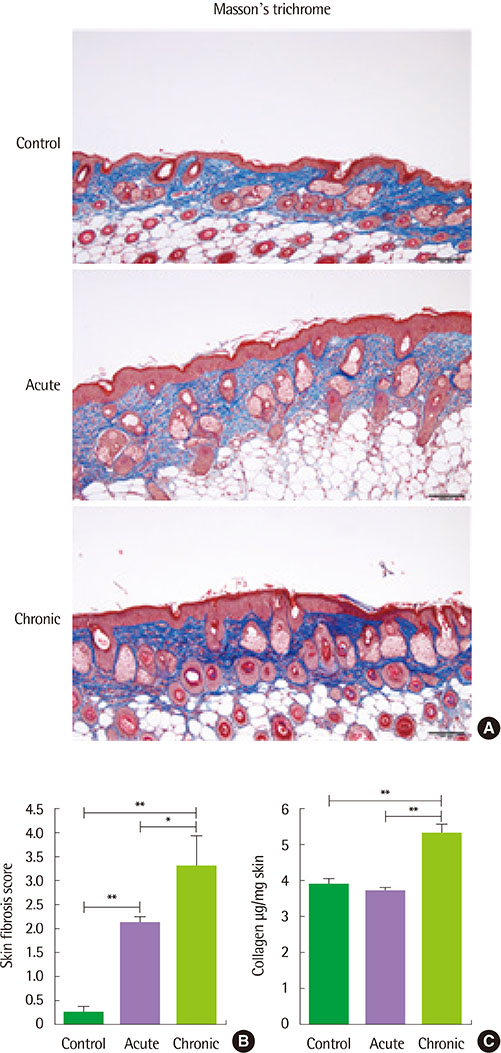
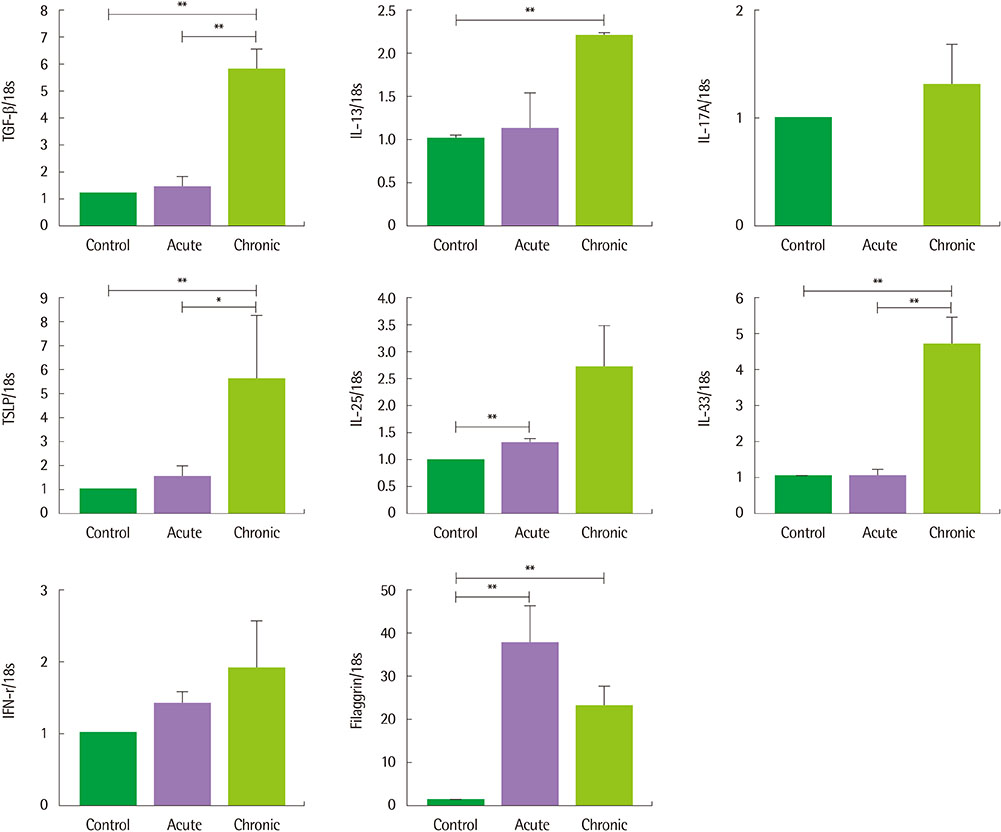
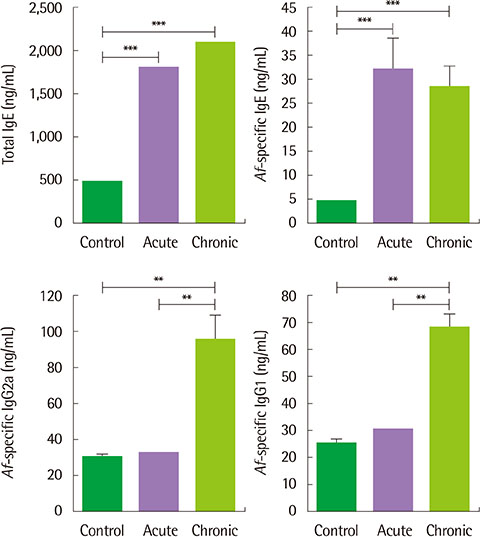
The clinical score and transepidermal water loss were more increased in the chronic AD model than in the acute AD model. Histologic findings showed that more increased epidermal thickness, neutrophil infiltration and hyperkeratosis in the chronic model than in the acute model. Skin fibrosis was more prominent in the chronic model than in the acute model. The mRNA expression levels of transforming growth factor (TGF)-β, thymic stromal lymphopoietin, and interleukin-33 were increased in the skin of the chronic model compared to the acute model. The levels of total IgE, Af-specific IgE, IgG1, and IgG2a were significantly increased in the chronic model compared to controls.
CONCLUSION
The Af-induced chronic AD model showed prominent fibrosis and increased TGF-β expression in the skin, which suggests that these models may be useful in the research for the mechanism of the chronic process in AD.
Keyword
MeSH Terms
-
Animals
Aspergillus fumigatus
Aspergillus*
Dermatitis, Atopic*
Fibrosis
Immunoglobulin E
Immunoglobulin G
Inflammation
Interleukin-33
Mice*
Neutrophil Infiltration
RNA, Messenger
Skin
Skin Diseases
Transforming Growth Factors
Water
Immunoglobulin E
Immunoglobulin G
Interleukin-33
RNA, Messenger
Transforming Growth Factors
Water
Figure
Cited by 1 articles
-
Atopic dermatitis mouse models
Hyunjung Kim
Allergy Asthma Respir Dis. 2019;7(3):113-115. doi: 10.4168/aard.2019.7.3.113.
Reference
-
1. Dokmeci E, Herrick CA. The immune system and atopic dermatitis. Semin Cutan Med Surg. 2008; 27:138–143.
Article2. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003; 112:6 Suppl. S118–S127.
Article3. DaVeiga SP. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc. 2012; 33:227–234.
Article4. Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin North Am. 2010; 30:269–280.
Article5. Oyoshi MK, He R, Kanaoka Y, ElKhal A, Kawamoto S, Lewis CN, et al. Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc Natl Acad Sci U S A. 2012; 109:4992–4997.
Article6. Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004; 113:651–657.
Article7. Yu J, Oh MH, Park JU, Myers AC, Dong C, Zhu Z, et al. Epicutaneous exposure to staphylococcal superantigen enterotoxin B enhances allergic lung inflammation via an IL-17A dependent mechanism. PLoS One. 2012; 7:e39032.
Article8. Breuer K, Kapp A, Werfel T. Bacterial infections and atopic dermatitis. Allergy. 2001; 56:1034–1041.
Article9. de Meer G, Janssen NA, Brunekreef B. Early childhood environment related to microbial exposure and the occurrence of atopic disease at school age. Allergy. 2005; 60:619–625.
Article10. Ibargoyen-Roteta N, Aguinaga-Ontoso I, Fernandez-Benitez M, Marin-Fernandez B, Guillen-Grima F, Serrano-Monzo I, et al. Role of the home environment in rhinoconjunctivitis and eczema in schoolchildren in Pamplona, Spain. J Investig Allergol Clin Immunol. 2007; 17:137–144.11. Miyake Y, Ohya Y, Tanaka K, Yokoyama T, Sasaki S, Fukushima W, et al. Home environment and suspected atopic eczema in Japanese infants: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2007; 18:425–432.
Article12. Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana Hershey GK, et al. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol. 2012; 130:639–644.e5.
Article13. Akei HS, Brandt EB, Mishra A, Strait RT, Finkelman FD, Warrier MR, et al. Epicutaneous aeroallergen exposure induces systemic TH2 immunity that predisposes to allergic nasal responses. J Allergy Clin Immunol. 2006; 118:62–69.
Article14. Matsushima H, Hayashi S, Shimada S. Skin scratching switches immune responses from Th2 to Th1 type in epicutaneously immunized mice. J Dermatol Sci. 2003; 32:223–230.
Article15. Heratizadeh A, Werfel T, Rösner LM. Adaptive immune response and associated trigger factors in atopic dermatitis. Hautarzt. 2015; 66:96–102.
Article16. Batista DI, Perez L, Orfali RL, Zaniboni MC, Samorano LP, Pereira NV, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015; 29:1091–1095.
Article17. Dhingra N, Guttman-Yassky E. A possible role for IL-17A in establishing Th2 inflammation in murine models of atopic dermatitis. J Invest Dermatol. 2014; 134:2071–2074.
Article18. Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003; 111:875–881.
Article19. Ishida Y, Kimura A, Kondo T, Hayashi T, Ueno M, Takakura N, et al. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol. 2007; 170:843–854.
Article20. Oh MH, Oh SY, Yu J, Myers AC, Leonard WJ, Liu YJ, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011; 186:7232–7242.
Article21. Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009; 129:742–751.
Article22. Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, et al. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol. 2008; 181:4311–4319.
Article23. McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013; 39:357–371.
Article24. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013; 210:2939–2950.
Article25. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013; 5:170ra16.
Article26. Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014; 111:367–372.
Article27. Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013; 110:13921–13926.
Article28. Kim RY, Rae B, Neal R, Donovan C, Pinkerton J, Balachandran L, et al. Elucidating novel disease mechanisms in severe asthma. Clin Transl Immunology. 2016; 5:e91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERRATUM: Correction of Funding Resource: Development of Aspergillus fumigatus-induced chronic atopic dermatitis mouse model
- Allergic March: Progression from Atopic Dermatitis to Asthma
- Innate Type 2 Response to Aspergillus fumigatus in a Murine Model of Atopic Dermatitis–like Skin Inflammation
- Regulation of Development in Aspergillus nidulans and Aspergillus fumigatus
- Chronic Toxoplasmosis Modulates the Induction of Contact Hypersensitivity by TNCB in Mouse Model