J Korean Med Sci.
2021 Oct;36(40):e261. 10.3346/jkms.2021.36.e261.
Innate Type 2 Response to Aspergillus fumigatus in a Murine Model of Atopic Dermatitis–like Skin Inflammation
- Affiliations
-
- 1Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea
- 2Department of Pediatrics, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
- 3Department of Medical Science, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Pediatrics, Mediplex Sejong Hospital, Incheon, Korea
- 5Department of Pediatrics, Pusan National University Yangsan Hospital, Yangsan, Korea
- 6Department of Pharmaceutical Engineering, College of Medical Sciences and Department of Medical Sciences, General Graduate School, Soon Chun Hyang University, Asan, Korea
- 7Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2521427
- DOI: http://doi.org/10.3346/jkms.2021.36.e261
Abstract
- Background
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease mediated by T helper type 2 (Th2) cells in acute phase. Group 2 innate lymphoid cells (ILCs) play a role in the initiation of the Th2 response. Although mold exposure is associated with the development of AD, studies on the underlying mechanisms are lacking. This study investigated whether group 2 ILCs are involved in inflammation in AD-like skin induced by Aspergillus fumigatus (Af).
Methods
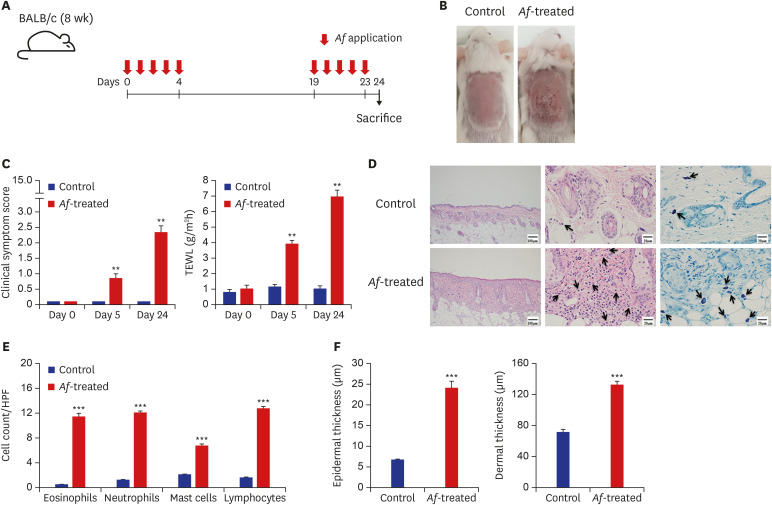
We investigated changes of group 2 ILCs population in Af-induced AD-like skin lesions. To induce AD-like skin lesions, Af extracts were applied to the dorsal skin of BALB/c and Rag1−/− mice five times per week, with repeat exposures at 2-week intervals.
Results
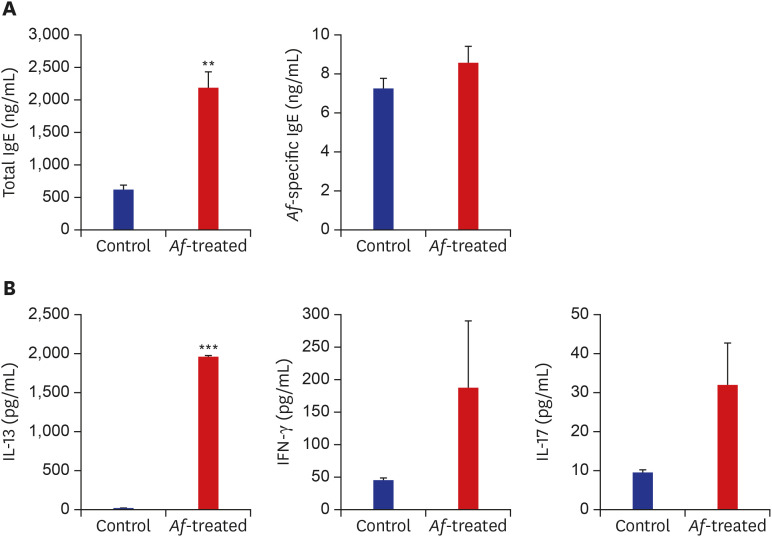
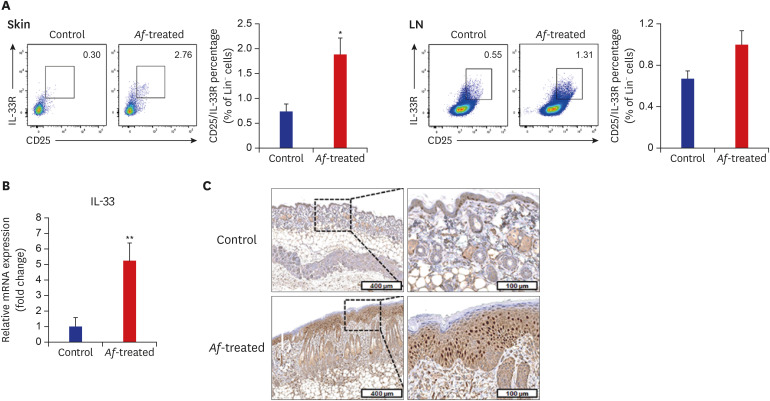
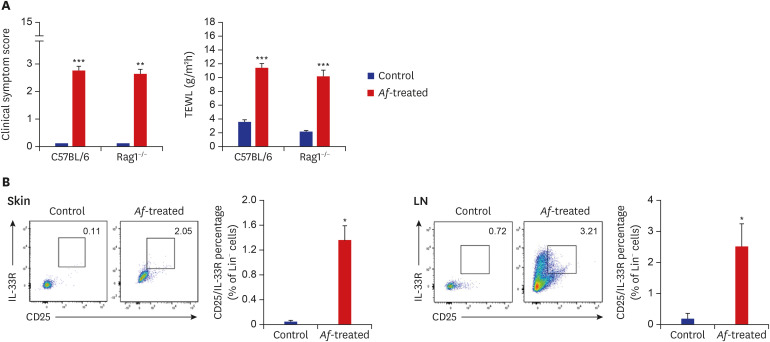
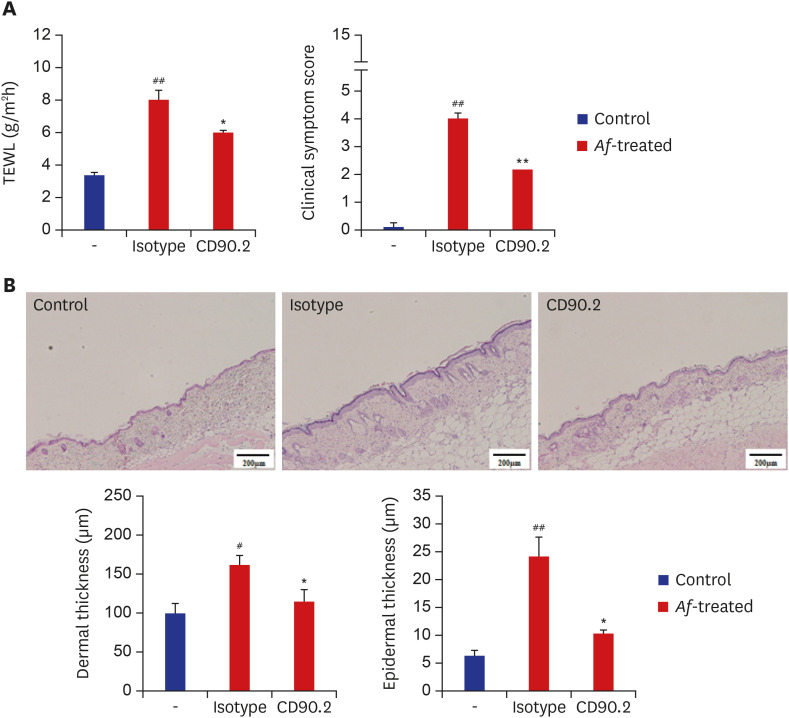
The clinical parameters were higher in the Af-treated group than in the control group. Histologic findings revealed epiderrmal and dermal thickening as well as eosinophil and mast cell infiltration into the skin of Af-treated mice. Populations of group 2 ILCs in the skin were also significantly higher in the Af-treated group. In addition, interleukin-33 mRNA expression was significantly higher in the skin lesions of the Af-treated mice. In the Rag1−/− mice lacking mature lymphocytes, AD-like skin lesions were still induced by Af and ILCs depletion using an anti-CD90.2 mAb lowered the Af-induced inflammatory response.
Conclusions
Group 2 ILCs may play a role in a murine model of Af-induced AD-like skin lesions.
Keyword
Figure
Cited by 1 articles
-
Personal Exposure to Total VOC is Associated With Symptoms of Atopic Dermatitis in Schoolchildren
Eun Kyo Ha, Ju Hee Kim, Dawon Park, Eun Lee, Seung Won Lee, Hye Mi Jee, Youn Ho Shin, Man Yong Han
J Korean Med Sci. 2022;37(8):e63. doi: 10.3346/jkms.2022.37.e63.
Reference
-
2. Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med. 2005; 352(22):2314–2324. PMID: 15930422.3. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010; 463(7280):540–544. PMID: 20023630.4. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010; 464(7293):1367–1370. PMID: 20200518.
Article5. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013; 5(170):170ra16.
Article6. Barlow JL, McKenzie AN. Type-2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol. 2014; 14(5):397–403. PMID: 25115682.
Article7. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001; 15(6):985–995. PMID: 11754819.
Article8. Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010; 107(43):18581–18586. PMID: 20937871.
Article9. Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013; 14(6):536–542. PMID: 23685824.
Article10. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013; 210(13):2939–2950. PMID: 24323357.
Article11. Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013; 110(34):13921–13926. PMID: 23918359.
Article12. Platt SD, Martin CJ, Hunt SM, Lewis CW. Damp housing, mould growth, and symptomatic health state. BMJ. 1989; 298(6689):1673–1678. PMID: 2503174.
Article13. Lee JY, Seo JH, Kwon JW, Yu J, Kim BJ, Lee SY, et al. Exposure to gene-environment interactions before 1 year of age may favor the development of atopic dermatitis. Int Arch Allergy Immunol. 2012; 157(4):363–371. PMID: 22123373.
Article14. Akei HS, Brandt EB, Mishra A, Strait RT, Finkelman FD, Warrier MR, et al. Epicutaneous aeroallergen exposure induces systemic TH2 immunity that predisposes to allergic nasal responses. J Allergy Clin Immunol. 2006; 118(1):62–69. PMID: 16815139.
Article15. Agarwal R, Gupta D. Severe asthma and fungi: current evidence. Med Mycol. 2011; 49(Suppl 1):S150–S157. PMID: 20662637.
Article16. Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012; 336(6086):1321–1325. PMID: 22674331.
Article17. Karvonen AM, Hyvärinen A, Korppi M, Haverinen-Shaughnessy U, Renz H, Pfefferle PI, et al. Moisture damage and asthma: a birth cohort study. Pediatrics. 2015; 135(3):e598–e606. PMID: 25687143.
Article18. Sharpe RA, Thornton CR, Tyrrell J, Nikolaou V, Osborne NJ. Variable risk of atopic disease due to indoor fungal exposure in NHANES 2005–2006. Clin Exp Allergy. 2015; 45(10):1566–1578. PMID: 25845975.
Article19. Nissen D, Petersen LJ, Esch R, Svejgaard E, Skov PS, Poulsen LK, et al. IgE-sensitization to cellular and culture filtrates of fungal extracts in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 1998; 81(3):247–255. PMID: 9759803.
Article20. Buentke E, Heffler LC, Wallin RP, Löfman C, Ljunggren HG, Scheynius A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin Exp Allergy. 2001; 31(10):1583–1593. PMID: 11678859.21. Johansson C, Eshaghi H, Linder MT, Jakobson E, Scheynius A. Positive atopy patch test reaction to Malassezia furfur in atopic dermatitis correlates with a T helper 2-like peripheral blood mononuclear cells response. J Invest Dermatol. 2002; 118(6):1044–1051. PMID: 12060401.
Article22. Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana Hershey GK, et al. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol. 2012; 130(3):639–644.e5. PMID: 22789397.
Article23. Mashiko S, Mehta H, Bissonnette R, Sarfati M. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J Dermatol Sci. 2017; 88(2):167–174. PMID: 28743611.
Article24. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016; 17(7):765–774. PMID: 27328006.
Article25. Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013; 14(6):564–573. PMID: 23603794.
Article26. Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12(11):1055–1062. PMID: 21909091.
Article27. Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, Kim KE. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010; 21(2 Pt 2):e457–e460. PMID: 20444170.
Article28. Lee HC, Headley MB, Loo YM, Berlin A, Gale M Jr, Debley JS, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012; 130(5):1187–1196.e5. PMID: 22981788.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Aspergillus fumigatus-induced chronic atopic dermatitis mouse model
- Type 2 Innate Lymphoid Cells and Skin Fibrosis in a Murine Model of Atopic Dermatitis-Like Skin Inflammation
- Cathelicidin LL-37: An Antimicrobial Peptide with a Role in Inflammatory Skin Disease
- Sphingolipids and Antimicrobial Peptides: Function and Roles in Atopic Dermatitis
- Aspergillus fumigatus infection in an ostrich with an enlarged neck due to respiratory problems