J Korean Med Sci.
2024 Aug;39(30):e221. 10.3346/jkms.2024.39.e221.
Type 2 Innate Lymphoid Cells and Skin Fibrosis in a Murine Model of Atopic Dermatitis-Like Skin Inflammation
- Affiliations
-
- 1Department of Pediatrics, Chung-Ang University College of Medicine, Seoul, Korea
- 2Clinical Trial Support Team, Chung-Ang University Gwangmyeong Hospital, Gwangmyeong, Korea
- 3Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea
- 4Department of Cell and Genetic Engineering, University of Ulsan College of Medicine, Seoul, Korea
- 5Center for Cell Therapy, Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea
- 6Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2558519
- DOI: http://doi.org/10.3346/jkms.2024.39.e221
Abstract
- Background
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease. Although murine studies have demonstrated that type 2 innate lymphoid cells (ILCs) mediate type 2 skin inflammation, their role in skin fibrosis in AD remains unclear. This study investigated whether type 2 ILCs are involved in skin fibrosis using an AD-like murine model.
Methods
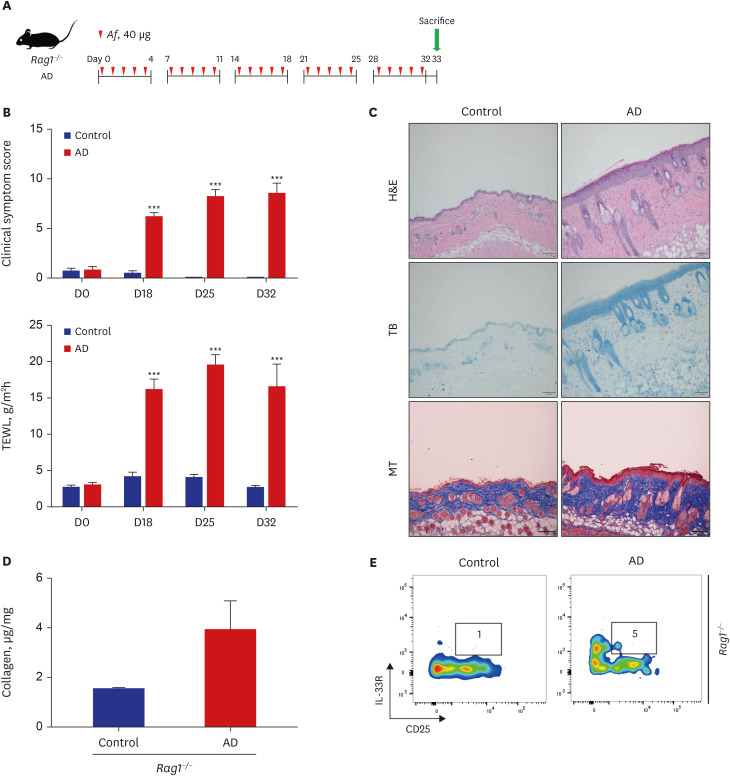
C57BL/6 mice were treated epicutaneously with Aspergillus fumigatus (Af) for 5 consecutive days per week for 5 weeks to induce skin fibrosis. Mature lymphocyte deficient Rag1−/− mice were also used to investigate the role of type 2 ILCs in skin fibrosis.
Results
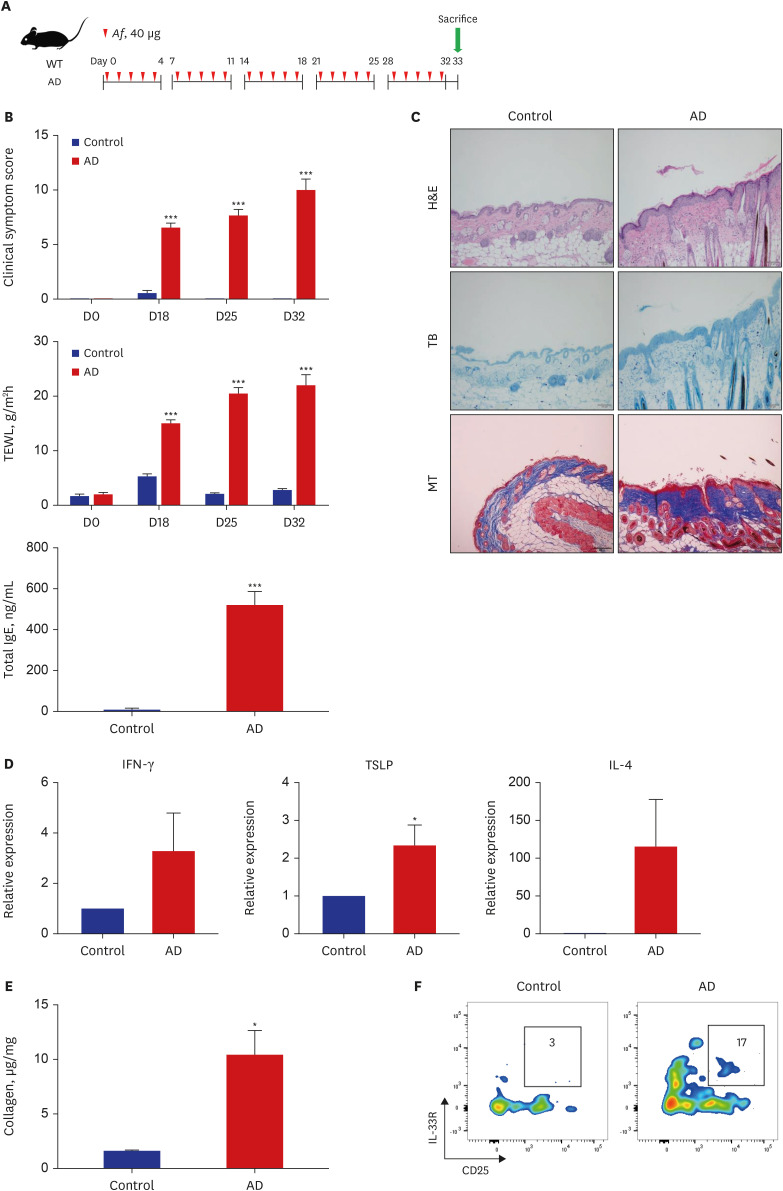
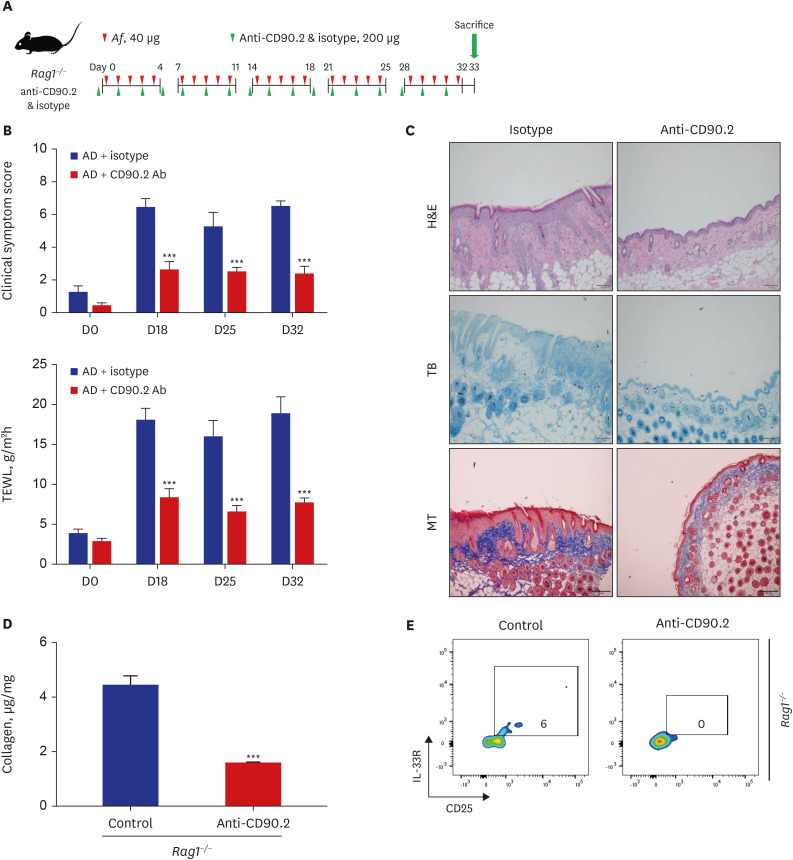
The clinical score and transepidermal water loss (TEWL) were significantly higher in the AD group than in the control group. The AD group also showed significantly increased epidermal and dermal thicknesses and significantly higher numbers of eosinophils, neutrophils, mast cells, and lymphocytes in the lesional skin than the control group. The lesional skin of the AD group showed increased stain of collagen and significantly higher levels of collagen than the control group (10.4 ± 2.2 µg/mg vs. 1.6 ± 0.1 µg/mg, P < 0.05). The AD group showed significantly higher populations of type 2 ILCs in the lesional skin compared to the control group (0.08 ± 0.01% vs. 0.03 ± 0.01%, P < 0.05). These findings were also similar with the AD group of Rag1−/− mice compared to their control group. Depletion of type 2 ILCs with anti-CD90.2 monoclonal antibodies significantly improved clinical symptom score, TEWL, and infiltration of inflammatory cells, and significantly decreased levels of collagen were observed in the AD group of Rag1−/− mice (1.6 ± 0.0 μg/mg vs. 4.5 ± 0.3 μg/mg, P < 0.001).
Conclusion
In the Af-induced AD-like murine model, type 2 ILCs were elevated, with increased levels of collagen. Additionally, removal of type 2 ILCs resulted in decreased collagen levels and improved AD-like pathological findings. These findings suggest that type 2 ILCs play a role in the mechanism of skin fibrosis in AD.
Figure
Reference
-
1. Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004; 113(5):651–657. PMID: 14991059.2. Whiteley J, Emir B, Seitzman R, Makinson G. The burden of atopic dermatitis in US adults: results from the 2013 National Health and Wellness Survey. Curr Med Res Opin. 2016; 32(10):1645–1651. PMID: 27240604.3. Bieber T, D’Erme AM, Akdis CA, Traidl-Hoffmann C, Lauener R, Schäppi G, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: where are we, and where should we go? J Allergy Clin Immunol. 2017; 139(4S):S58–S64. PMID: 28390478.4. Jeon YH, Ahn K, Kim J, Shin M, Hong SJ, Lee SY, et al. Clinical characteristics of atopic dermatitis in Korean school-aged children and adolescents according to onset age and severity. J Korean Med Sci. 2022; 37(4):e30. PMID: 35075829.5. Kim DH, Li K, Seo SJ, Jo SJ, Yim HW, Kim CM, et al. Quality of life and disease severity are correlated in patients with atopic dermatitis. J Korean Med Sci. 2012; 27(11):1327–1332. PMID: 23166413.6. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009; 9(5):437–446. PMID: 19550302.7. Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011; 2(3):110. PMID: 21994899.8. Tsoi LC, Rodriguez E, Stölzl D, Wehkamp U, Sun J, Gerdes S, et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J Allergy Clin Immunol. 2020; 145(5):1406–1415. PMID: 31891686.9. Rankin AL, Mumm JB, Murphy E, Turner S, Yu N, McClanahan TK, et al. IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol. 2010; 184(3):1526–1535. PMID: 20042577.10. Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, et al. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol. 2008; 181(6):4311–4319. PMID: 18768889.11. Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009; 129(3):742–751. PMID: 18830273.12. Demehri S, Turkoz A, Kopan R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell. 2009; 16(1):55–66. PMID: 19573812.13. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010; 463(7280):540–544. PMID: 20023630.14. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010; 464(7293):1367–1370. PMID: 20200518.15. Kim HY. Innate immunity in asthma. Allergy Asthma Respir Dis. 2014; 2(5):317–325.16. Park A, Lee E, Park H, Park MN, Lee J, Song KB, et al. Innate type 2 response to Aspergillus fumigatus in a murine model of atopic dermatitis-like skin inflammation. J Korean Med Sci. 2021; 36(40):e261. PMID: 34664800.17. Zhang J, Qiu J, Zhou W, Cao J, Hu X, Mi W, et al. Neuropilin-1 mediates lung tissue-specific control of ILC2 function in type 2 immunity. Nat Immunol. 2022; 23(2):237–250. PMID: 35075279.18. Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014; 111(1):367–372. PMID: 24344271.19. Park A, Park H, Yu J. Development of Aspergillus fumigatus-induced chronic atopic dermatitis mouse model. Allergy Asthma Respir Dis. 2019; 7(3):150–157.20. Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012; 336(6086):1321–1325. PMID: 22674331.21. Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013; 14(6):564–573. PMID: 23603794.22. Imai Y, Yasuda K, Nagai M, Kusakabe M, Kubo M, Nakanishi K, et al. IL-33-Induced Atopic Dermatitis-Like Inflammation in Mice Is Mediated by Group 2 Innate Lymphoid Cells in Concert with Basophils. J Invest Dermatol. 2019; 139(10):2185–2194.e3. PMID: 31121178.23. Bartemes KR, Kita H. Roles of innate lymphoid cells (ILCs) in allergic diseases: the 10-year anniversary for ILC2s. J Allergy Clin Immunol. 2021; 147(5):1531–1547. PMID: 33965091.24. Leyva-Castillo JM, Galand C, Mashiko S, Bissonnette R, McGurk A, Ziegler SF, et al. ILC2 activation by keratinocyte-derived IL-25 drives IL-13 production at sites of allergic skin inflammation. J Allergy Clin Immunol. 2020; 145(6):1606–1614.e4. PMID: 32179159.25. Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013; 5(170):170ra16.26. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013; 210(13):2939–2950. PMID: 24323357.27. Ebbo M, Crinier A, Vély F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017; 17(11):665–678. PMID: 28804130.28. Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J Allergy Clin Immunol. 2016; 138(3):814–824.e11. PMID: 27156176.29. Hong JY, Bentley JK, Chung Y, Lei J, Steenrod JM, Chen Q, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014; 134(2):429–439. PMID: 24910174.30. Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011; 12(7):631–638. PMID: 21623379.31. Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012; 303(7):L577–L588. PMID: 22865552.32. Batista DI, Perez L, Orfali RL, Zaniboni MC, Samorano LP, Pereira NV, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015; 29(6):1091–1095. PMID: 25271795.33. Dhingra N, Guttman-Yassky E. A possible role for IL-17A in establishing Th2 inflammation in murine models of atopic dermatitis. J Invest Dermatol. 2014; 134(8):2071–2074. PMID: 25029321.34. Oh MH, Oh SY, Yu J, Myers AC, Leonard WJ, Liu YJ, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011; 186(12):7232–7242. PMID: 21576506.35. Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003; 111(4):875–881. PMID: 12704372.36. Ishida Y, Kimura A, Kondo T, Hayashi T, Ueno M, Takakura N, et al. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol. 2007; 170(3):843–854. PMID: 17322370.37. Nakatsuka Y, Yaku A, Handa T, Vandenbon A, Hikichi Y, Motomura Y, et al. Profibrotic function of pulmonary group 2 innate lymphoid cells is controlled by regnase-1. Eur Respir J. 2021; 57(3):2000018. PMID: 32978308.38. Starkey MR, McKenzie AN, Belz GT, Hansbro PM. Pulmonary group 2 innate lymphoid cells: surprises and challenges. Mucosal Immunol. 2019; 12(2):299–311. PMID: 30664706.39. Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007; 35(Pt 4):661–664. PMID: 17635115.40. Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DM, Artis D. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A. 2015; 112(34):10762–10767. PMID: 26243875.41. McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013; 39(2):357–371. PMID: 23954132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Innate Type 2 Response to Aspergillus fumigatus in a Murine Model of Atopic Dermatitis–like Skin Inflammation
- Cathelicidin LL-37: An Antimicrobial Peptide with a Role in Inflammatory Skin Disease
- Development of Aspergillus fumigatus-induced chronic atopic dermatitis mouse model
- A Case of Atopic Dermatitis with Psoriasis
- Sphingolipids and Antimicrobial Peptides: Function and Roles in Atopic Dermatitis