J Periodontal Implant Sci.
2019 Jun;49(3):138-147. 10.5051/jpis.2019.49.3.138.
Helicobacter pylori inhibited cell proliferation in human periodontal ligament fibroblasts through the Cdc25C/CDK1/cyclinB1 signaling cascade
- Affiliations
-
- 1Department of Stomatology, Nanfang Hospital, Southern Medical University, Guangzhou, China. zhaowh@smu.edu.cn
- KMID: 2454084
- DOI: http://doi.org/10.5051/jpis.2019.49.3.138
Abstract
- PURPOSE
Several studies have shown that the oral cavity is a secondary location for Helicobacter pylori colonization and that H. pylori is associated with the severity of periodontitis. This study investigated whether H. pylori had an effect on the periodontium. We established an invasion model of a standard strain of H. pylori in human periodontal ligament fibroblasts (hPDLFs), and evaluated the effects of H. pylori on cell proliferation and cell cycle progression.
METHODS
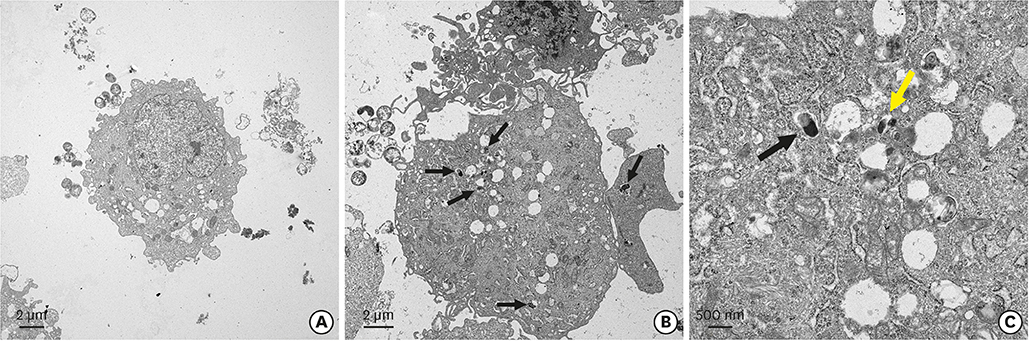
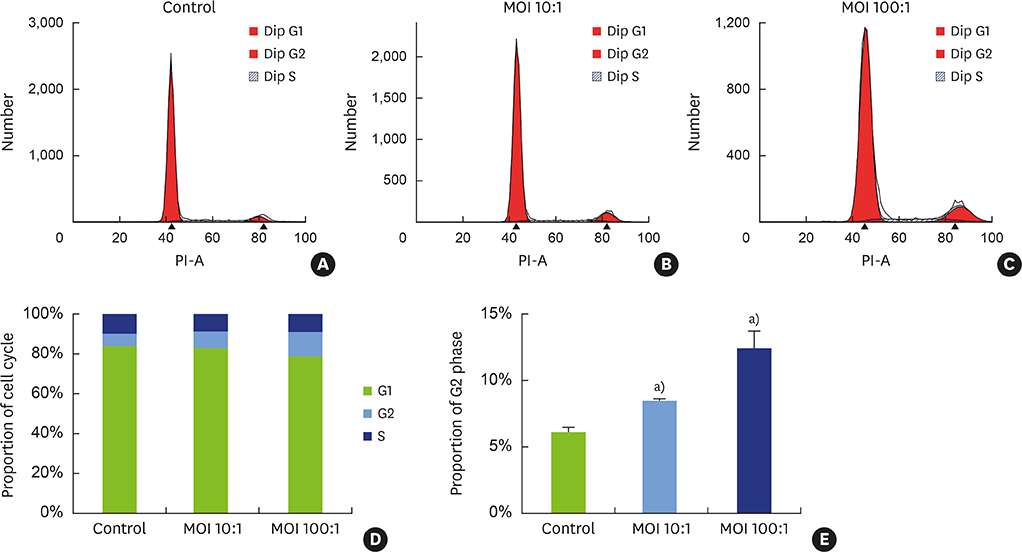
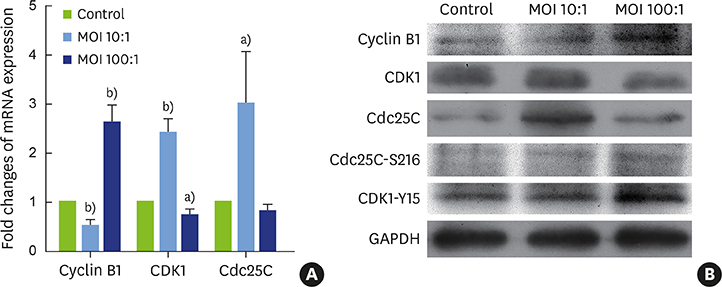
Different concentrations of H. pylori were used to infect hPDLFs, with 6 hours of co-culture. The multiplicity of infection in the low- and high-concentration groups was 10:1 and 100:1, respectively. The Cell Counting Kit-8 method and Ki-67 immunofluorescence were used to detect cell proliferation. Flow cytometry, quantitative real-time polymerase chain reaction, and western blots were used to detect cell cycle progression. In the high-concentration group, the invasion of H. pylori was observed by transmission electron microscopy.
RESULTS
It was found that H. pylori invaded the fibroblasts, with cytoplasmic localization. Analyses of cell proliferation and flow cytometry showed that H. pylori inhibited the proliferation of periodontal fibroblasts by causing G2 phase arrest. The inhibition of proliferation and G2 phase arrest were more obvious in the high-concentration group. In the low-concentration group, the G2 phase regulatory factors cyclin dependent kinase 1 (CDK1) and cell division cycle 25C (Cdc25C) were upregulated, while cyclin B1 was inhibited. However, in the high-concentration group, cyclin B1 was upregulated and CDK1 was inhibited. Furthermore, the deactivated states of tyrosine phosphorylation of CDK1 (CDK1-Y15) and serine phosphorylation of Cdc25C (Cdc25C-S216) were upregulated after H. pylori infection.
CONCLUSIONS
In our model, H. pylori inhibited the proliferation of hPDLFs and exerted an invasive effect, causing G2 phase arrest via the Cdc25C/CDK1/cyclin B1 signaling cascade. Its inhibitory effect on proliferation was stronger in the high-concentration group.
MeSH Terms
-
Blotting, Western
CDC2 Protein Kinase
Cell Count
Cell Cycle
Cell Proliferation*
Coculture Techniques
Colon
Cyclin B1
Cytoplasm
Fibroblasts*
Flow Cytometry
Fluorescent Antibody Technique
G2 Phase
Helicobacter pylori*
Helicobacter*
Humans*
Methods
Microscopy, Electron, Transmission
Mouth
Periodontal Ligament*
Periodontitis
Periodontium
Phosphorylation
Real-Time Polymerase Chain Reaction
Serine
Tyrosine
CDC2 Protein Kinase
Cyclin B1
Serine
Tyrosine
Figure
Reference
-
1. Lina TT, Alzahrani S, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Immune evasion strategies used by Helicobacter pylori. World J Gastroenterol. 2014; 20:12753–12766.2. Yee JK. Helicobacter pylori colonization of the oral cavity: a milestone discovery. World J Gastroenterol. 2016; 22:641–648.
Article3. Krajden S, Fuksa M, Anderson J, Kempston J, Boccia A, Petrea C, et al. Examination of human stomach biopsies, saliva, and dental plaque for Campylobacter pylori . J Clin Microbiol. 1989; 27:1397–1398.
Article4. Dye BA, Kruszon-Moran D, McQuillan G. The relationship between periodontal disease attributes and Helicobacter pylori infection among adults in the United States. Am J Public Health. 2002; 92:1809–1815.
Article5. Souto R, Colombo AP. Detection of Helicobacter pylori by polymerase chain reaction in the subgingival biofilm and saliva of non-dyspeptic periodontal patients. J Periodontol. 2008; 79:97–103.
Article6. Al Asqah M, Al Hamoudi N, Anil S, Al Jebreen A, Al-Hamoudi WK. Is the presence of Helicobacter pylori in dental plaque of patients with chronic periodontitis a risk factor for gastric infection? Can J Gastroenterol. 2009; 23:177–179.
Article7. Nonnenmacher C, Dalpke A, Rochon J, Flores-de-Jacoby L, Mutters R, Heeg K. Real-time polymerase chain reaction for detection and quantification of bacteria in periodontal patients. J Periodontol. 2005; 76:1542–1549.
Article8. Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005; 49:491–516.
Article9. Mysak J, Podzimek S, Sommerova P, Lyuya-Mi Y, Bartova J, Janatova T, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014; 2014:476068.10. Socransky SS, Haffajee AD. Dental biofilms difficult therapeutic targets. Periodontol 2000. 2002; 28:12–55.
Article11. Andersen RN, Ganeshkumar N, Kolenbrander PE. Helicobacter pylori adheres selectively to Fusobacterium spp. Oral Microbiol Immunol. 1998; 13:51–54.
Article12. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998; 25:134–144.
Article13. Okuda K, Kimizuka R, Katakura A, Nakagawa T, Ishihara K. Ecological and immunopathological implications of oral bacteria in Helicobacter pylori-infected disease. J Periodontol. 2003; 74:123–128.
Article14. Hu Z, Zhang Y, Li Z, Yu Y, Kang W, Han Y, et al. Effect of Helicobacter pylori infection on chronic periodontitis by the change of microecology and inflammation. Oncotarget. 2016; 7:66700–66712.15. Al Sayed A, Anand PS, Kamath KP, Patil S, Preethanath RS, Anil S. Oral cavity as an extragastric reservoir of Helicobacter pylori . ISRN Gastroenterol. 2014; 2014:261369.16. Zu L. Reviews of research on the relationship between oral Helicobacter pylori and H. pylori infection. Infect Int. 2016; 5:5–10.
Article17. Salehi MR, Shah Aboei M, Naghsh N, Hajisadeghi S, Ajami E. A comparison in prevalence of Helicobacter pylori in the gingival crevicular fluid from subjects with periodontitis and healthy individuals using polymerase chain reaction. J Dent Res Dent Clin Dent Prospect. 2013; 7:238–243.18. Chu YT, Wang YH, Wu JJ, Lei HY. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010; 78:4157–4165.
Article19. Evans DG, Evans DJ Jr, Graham DY. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology. 1992; 102:1557–1567.
Article20. Dubois A, Borén T. Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 2007; 9:1108–1116.
Article21. Huang Y, Wang QL, Cheng DD, Xu WT, Lu NH. Adhesion and invasion of gastric mucosa epithelial cells by Helicobacter pylori . Front Cell Infect Microbiol. 2016; 6:159.
Article22. Petersen AM, Krogfelt KA. Helicobacter pylori: an invading microorganism? A review. FEMS Immunol Med Microbiol. 2003; 36:117–126.23. Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, et al. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori . Gastroenterology. 2007; 132:1009–1023.
Article24. Amieva MR, Salama NR, Tompkins LS, Falkow S. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell Microbiol. 2002; 4:677–690.
Article25. Bode G, Mauch F, Malfertheiner P. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol Infect. 1993; 111:483–490.26. Cuddihy AR, O'Connell MJ. Cell-cycle responses to DNA damage in G2. Int Rev Cytol. 2003; 222:99–140.
Article27. Strauss B, Harrison A, Coelho PA, Yata K, Zernicka-Goetz M, Pines J. Cyclin B1 is essential for mitosis in mouse embryos, and its nuclear export sets the time for mitosis. J Cell Biol. 2018; 217:179–193.
Article28. Santos SD, Wollman R, Meyer T, Ferrell JE Jr. Spatial positive feedback at the onset of mitosis. Cell. 2012; 149:1500–1513.
Article29. Schmidt M, Rohe A, Platzer C, Najjar A, Erdmann F, Sippl W. Regulation of G2/M transition by inhibition of WEE1 and PKMYT1 kinases. Molecules. 2017; 22:E2045.
Article30. Sur S, Agrawal DK. Phosphatases and kinases regulating CDC25 activity in the cell cycle: clinical implications of CDC25 overexpression and potential treatment strategies. Mol Cell Biochem. 2016; 416:33–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of high glucose on the prostaglandin E2 production in human gingival fibroblasts and periodontal ligament cells
- Perspective of Helicobacter pylori Research: Molecular Pathogenesis of Helicobacter pylori Virulence Factors
- Effect of the Electrical Stimulation on the Human Periodontal Ligament Cells and Gingival Fibroblasts
- Effect of Inorganic Polyphosphate on Cultured Periodontal Ligament Cells
- Effects of some herbal drugs on gingival fibroblast and periodontal ligament cellular activity