J Bacteriol Virol.
2018 Dec;48(4):156-165. 10.4167/jbv.2018.48.4.156.
Inhibitory Effect of Ginsenosides Rh1 and Rg2 on Oxidative Stress in LPS-Stimulated RAW 264.7 Cells
- Affiliations
-
- 1College of Pharmacy and Institute of Drug Research and Development, Chungnam National University, Daejeon, Korea. kheo@cnu.ac.kr
- 2Shine & Shine, Korea Research Institute of Bioscience and Biotechnology (KRIBB), BCV 118, Daejeon, Korea.
- KMID: 2452068
- DOI: http://doi.org/10.4167/jbv.2018.48.4.156
Abstract
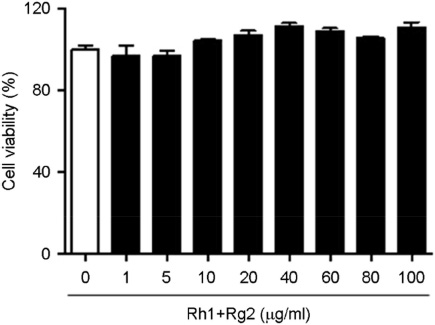
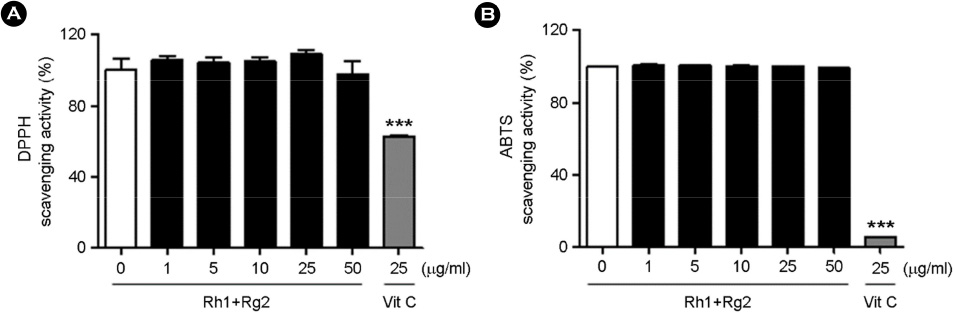
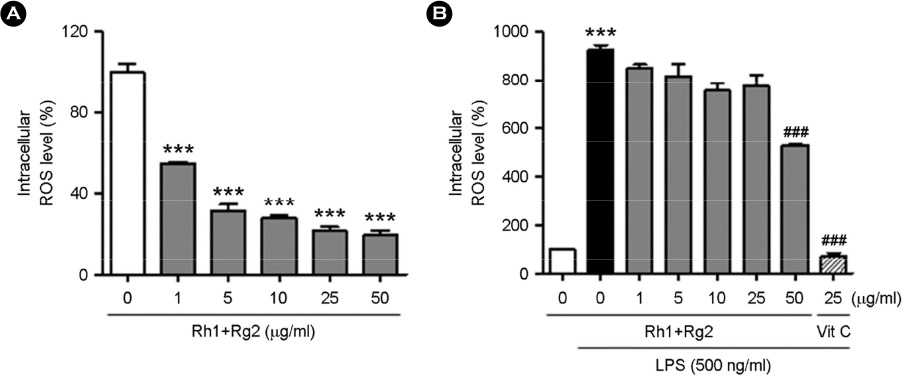
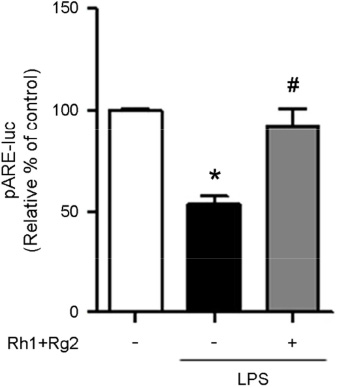
- Minor ginsenosides Rh1 and Rg2 were isolated from Korean red ginseng and reported to have various biological effects on anti-inflammatory and anti-stress activities. However, the effects of Rh1 and Rg2 on antioxidant activity and their regulatory effects on the antioxidant enzymes have not been studied. Since oxidative stress is one of the major toxic inflammatory responses stimulated by lipopolysaccharides (LPS), the present study investigated the role of minor ginsenosides Rh1 and Rg2 on antioxidant effects in LPS-treated RAW 264.7 cells. In this study, we found that treatment with ginsenosides Rh1 and Rg2 strongly inhibited LPS-stimulated intracellular ROS production in cells. Luciferase assay showed that treatment with LPS reduced antioxidant response element (ARE) encoding the pARE-luc promoter activity, while ginsenosides inhibited the pARE-luc promoter activity. Moreover, ginsenosides Rh1 and Rg2 exhibited anti-oxidative activity in LPS-induced cells by upregulating antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase. Our results suggest that minor ginsenosides Rh1 and Rg2 may be potential bio-active compounds for antioxidative effects by inhibiting the generation of ROS in RAW 264.7 cells.
Keyword
MeSH Terms
-
Antioxidant Response Elements
Antioxidants
Catalase
Ginsenosides*
Glutathione Peroxidase
Lipopolysaccharides
Luciferases
Oxidative Stress*
Panax
RAW 264.7 Cells*
Reactive Oxygen Species
Superoxide Dismutase
Antioxidants
Catalase
Ginsenosides
Glutathione Peroxidase
Lipopolysaccharides
Luciferases
Reactive Oxygen Species
Superoxide Dismutase
Figure
Cited by 1 articles
-
Correction: Inhibitory Effect of Ginsenosides Rh1 and Rg2 on Oxidative Stress in LPS-Stimulated RAW 264.7 Cells
Yujin Jin, Naehwan Baek, Soyoung Back, Chang-Seon Myung, Kyung-Sun Heo
J Bacteriol Virol. 2019;49(2):93-93. doi: 10.4167/jbv.2019.49.2.93.
Reference
-
1. Baek N, Sim S, Heo KS. LPS-stimulated macrophage activation affects endothelial dysfunction. J Bacteriol Virol. 2018; 48:23–30.
Article2. Bang S, Lee C, Ryu J, Li W, Koh YS, Jeon JH, et al. Simultaneous determination of the bioactive compounds from Sparassis crispa (Wulf.) by HPLC-DAD and their inhibitory effects on LPS-stimulated cytokine production in bone marrow-derived dendritic cell. Arch Pharm Res. 2018; 41:823–829.
Article3. Choi JS, Islam MN, Ali MY, Kim YM, Park HJ, Sohn HS, et al. The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer's disease, anti-diabetic, and anti-inflammatory activities. Arch Pharm Res. 2014; 37:1354–1363.
Article4. Chen Y, Zhou Z, Min W. Mitochondria, Oxidative Stress and Innate Immunity. Front Physiol. 2018; 9:1487.
Article5. Zhu X, Wang K, Zhou F, Zhu L. Paeoniflorin attenuates atRAL-induced oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress in retinal pigment epithelial cells via triggering Ca(2+)/CaMKII-dependent activation of AMPK. Arch Pharm Res. 2018; 41:1009–1018.
Article6. Vitkov L, Hannig M, Minnich B, Herrmann M. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity. 2018; 51:304–309.
Article7. Bombaca ACS, Viana PG, Santos ACC, Silva TL, Rodrigues ABM, Guimarães ACR, et al. Mitochondrial disfunction and ROS production are essential for anti-Trypanosoma cruzi activity of beta-lapachone-derived naphthoimidazoles. Free Radic Biol Med. 2018; 130:408–418.8. Yu JW, Lee MS. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res. 2016; 39:1503–1518.
Article9. Zhang Y. Cell toxicity mechanism and biomarker. Clin Transl Med. 2018; 7:34.
Article10. Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013; 65:1174–1194.
Article11. Erickson AM, Nevarea Z, Gipp JJ, Mulcahy RT. Identification of a variant antioxidant response element in the promoter of the human glutamate-cysteine ligase modifier subunit gene. J Biol Chem. 2002; 277:30730–30737.
Article12. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999; 274:26071–26078.
Article13. Nguyen T, Pickett CB. Regulation of rat glutathione S-transferase Ya subunit gene expression. DNA-protein interaction at the antioxidant responsive element. J Biol Chem. 1992; 267:13535–13539.
Article14. Bahia PK, Rattray M, Williams RJ. Dietary flavonoid (−)epicatechin stimulates phosphatidylinositol 3-kinase-dependent anti-oxidant response element activity and up-regulates glutathione in cortical astrocytes. J Neurochem. 2008; 106:2194–2204.15. Song BK, Kim KM, Choi KD, Im WT. Production of the Rare Ginsenoside Rh2-MIX (20(S)-Rh2, 20(R)-Rh2, Rk2, and Rh3) by Enzymatic Conversion Combined with Acid Treatment and Evaluation of Its Anti-Cancer Activity. J Microbiol Biotechnol. 2017; 27:1233–1241.
Article16. Cui CH, Kim DJ, Jung SC, Kim SC, Im WT. Enhanced Production of Gypenoside LXXV Using a Novel Ginsenoside-Transforming beta-Glucosidase from Ginseng-Cultivating Soil Bacteria and Its Anti-Cancer Property. Molecules. 2017; 22:pii: E844.17. Baatar D, Siddiqi MZ, Im WT, Ul Khaliq N, Hwang SG. Anti-Inflammatory Effect of Ginsenoside Rh2-Mix on Lipopolysaccharide-Stimulated RAW 264.7 Murine Macrophage Cells. J Med Food. 2018; 21:951–960.
Article18. Jung SC, Kim W, Park SC, Jeong J, Park MK, Lim S, et al. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014; 55:2177–2188.
Article19. Samukawa K, Suzuki Y, Ohkubo N, Aoto M, Sakanaka M, Mitsuda N. Protective effect of ginsenosides Rg(2) and Rh(1) on oxidation-induced impairment of erythrocyte membrane properties. Biorheology. 2008; 45:689–700.
Article20. Jang H, Lee JW, Lee C, Jin Q, Lee MK, Lee CK, et al. Flavonol glycosides from the aerial parts of Gynostemma pentaphyllum and their antioxidant activity. Arch Pharm Res. 2016; 39:1232–1236.
Article21. Park HS, Quan KT, Han JH, Jung SH, Lee DH, Jo E, et al. Rubiarbonone C inhibits platelet-derived growth factor-induced proliferation and migration of vascular smooth muscle cells through the focal adhesion kinase, MAPK and STAT3 Tyr(705) signalling pathways. Br J Pharmacol. 2017; 174:4140–4154.
Article22. Park HS, Han JH, Jung SH, Lee DH, Heo KS, Myung CS. Anti-apoptotic effects of autophagy via ROS regulation in microtubule-targeted and PDGF-stimulated vascular smooth muscle cells. Korean J Physiol Pharmacol. 2018; 22:349–360.
Article23. Heo KS, Le NT, Cushman HJ, Giancursio CJ, Chang E, Woo CH, et al. Disturbed flow-activated p90RSK kinase accelerates atherosclerosis by inhibiting SENP2 function. J Clin Invest. 2015; 125:1299–1310.
Article24. Ravanfar SA, Karimi E, Mehrabanjoubani P, Ebrahimi M. Enhancement of phenolic and flavonoids compounds, antioxidant and cytotoxic effects in regenerated red cabbage by application of Zeatin. Nat Prod Res. 2018; 1–5.
Article25. Hoyos-Arbeláez J, Blandón-Naranjo L, Vázquez M, Contreras-Calderón J. Antioxidant capacity of mango fruit (Mangifera indica). An electrochemical study as an approach to the spectrophotometric methods. Food Chem. 2018; 266:435–440.
Article26. Huang CS, Chang LS, Anderson ME, Meister A. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1993; 268:19675–19680.
Article27. Cho RL, Yang CC, Tseng HC, Hsiao LD, Lin CC, Yang CM. Haem oxygenase-1 up-regulation by rosiglitazone via ROS-dependent Nrf2-antioxidant response elements axis or PPARgamma attenuates LPS-mediated lung inflammation. Br J Pharmacol. 2018; 175:3928–3946.
Article28. Farzaei MH, Zobeiri M, Parvizi F, El-Senduny FF, Marmouzi I, Coy-Barrera E, et al. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients. 2018; 10:pii: E855.
Article29. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011; 15:1583–1606.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correction: Inhibitory Effect of Ginsenosides Rh1 and Rg2 on Oxidative Stress in LPS-Stimulated RAW 264.7 Cells
- Lignans and Macrolides from the Leaves of Houttuynia cordata with Inhibitory Activity against NO Production in Murine Macrophage RAW 264.7 Cells
- Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells
- Anti-inflammatory activities of Scolopendra subspinipes mutilans in RAW 264.7 cells
- The effect of bile acids on Porphyromonas gingivalis lipopolysaccharide-induced inflammatory response