J Korean Acad Oral Health.
2020 Dec;44(4):205-213. 10.11149/jkaoh.2020.44.4.205.
The effect of bile acids on Porphyromonas gingivalis lipopolysaccharide-induced inflammatory response
- Affiliations
-
- 1Department of Preventive & Social Dentistry, School of Dentistry, Seoul National University, Seoul, Korea
- KMID: 2510251
- DOI: http://doi.org/10.11149/jkaoh.2020.44.4.205
Abstract
Objectives
In this study, I aimed to evaluate the inhibitory effect of bile acids on the inflammatory response and osteoclastogenesis in RAW 264.7 cells activated through lipopolysaccharide (LPS) and receptor activator of nuclear factor-kB ligand (RANKL) of the periodontal pathogen Porphyromonas gingivalis.
Methods
Myelomonocytic RAW 264.7 cells were activated through P. gingivalis LPS to induce inflammatory response, and were treated with three bile acids, including taurodeoxycholate, taurocholate, and glycocholate at different concentrations. The cytotoxicity of bile acids was assessed through the MTT assay. To evaluate the inhibitory effect of bile acids on inflammatory response, the induction levels of the pro-inflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-a were measured using ELISA 12 h after the treatment. Additionally, after activating the cells with RANKL to promote osteoclastogenesis, we examined whether bile acids suppressed osteoclast differentiation using the tartrate-resistant acid phosphatase staining.
Results
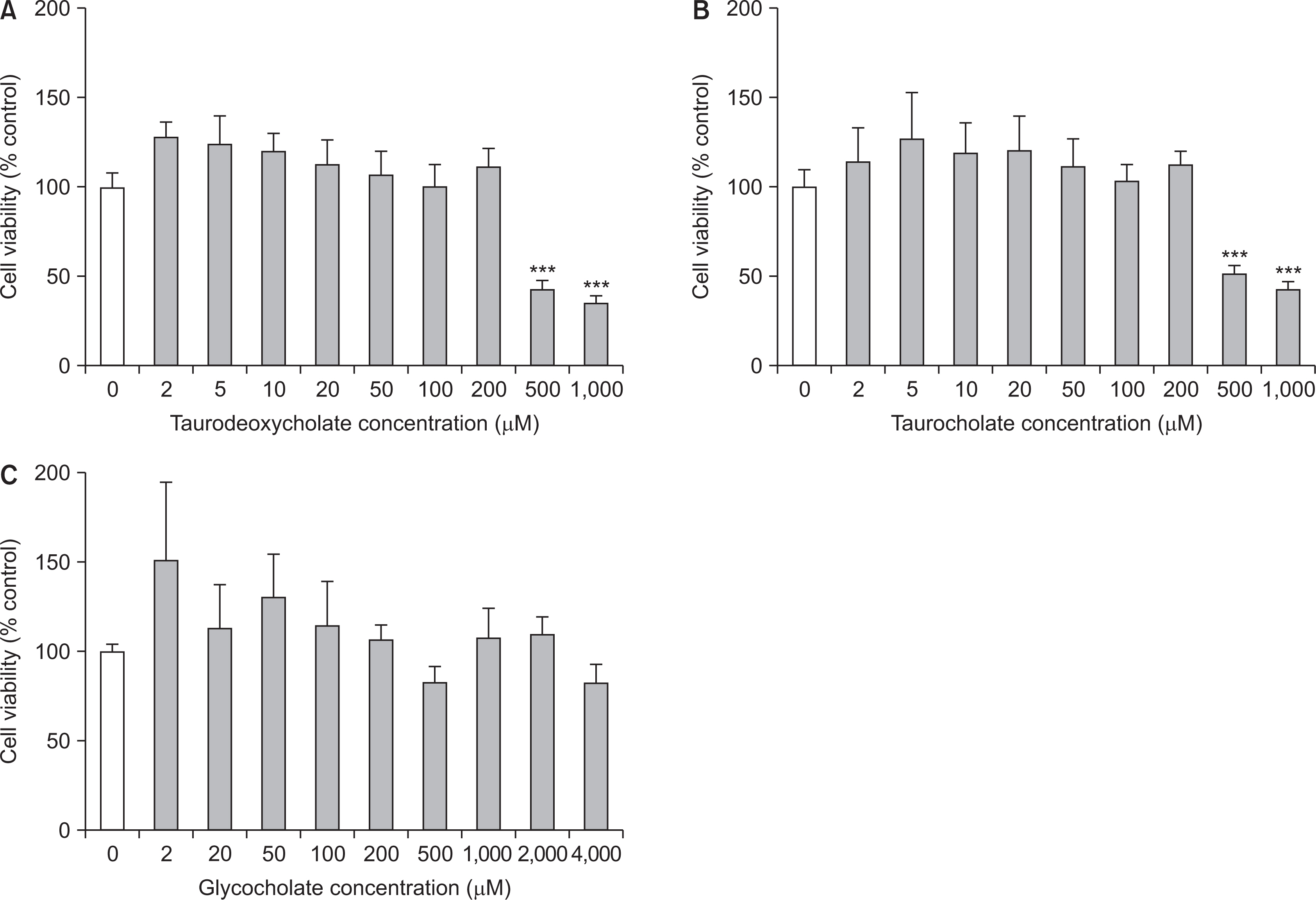
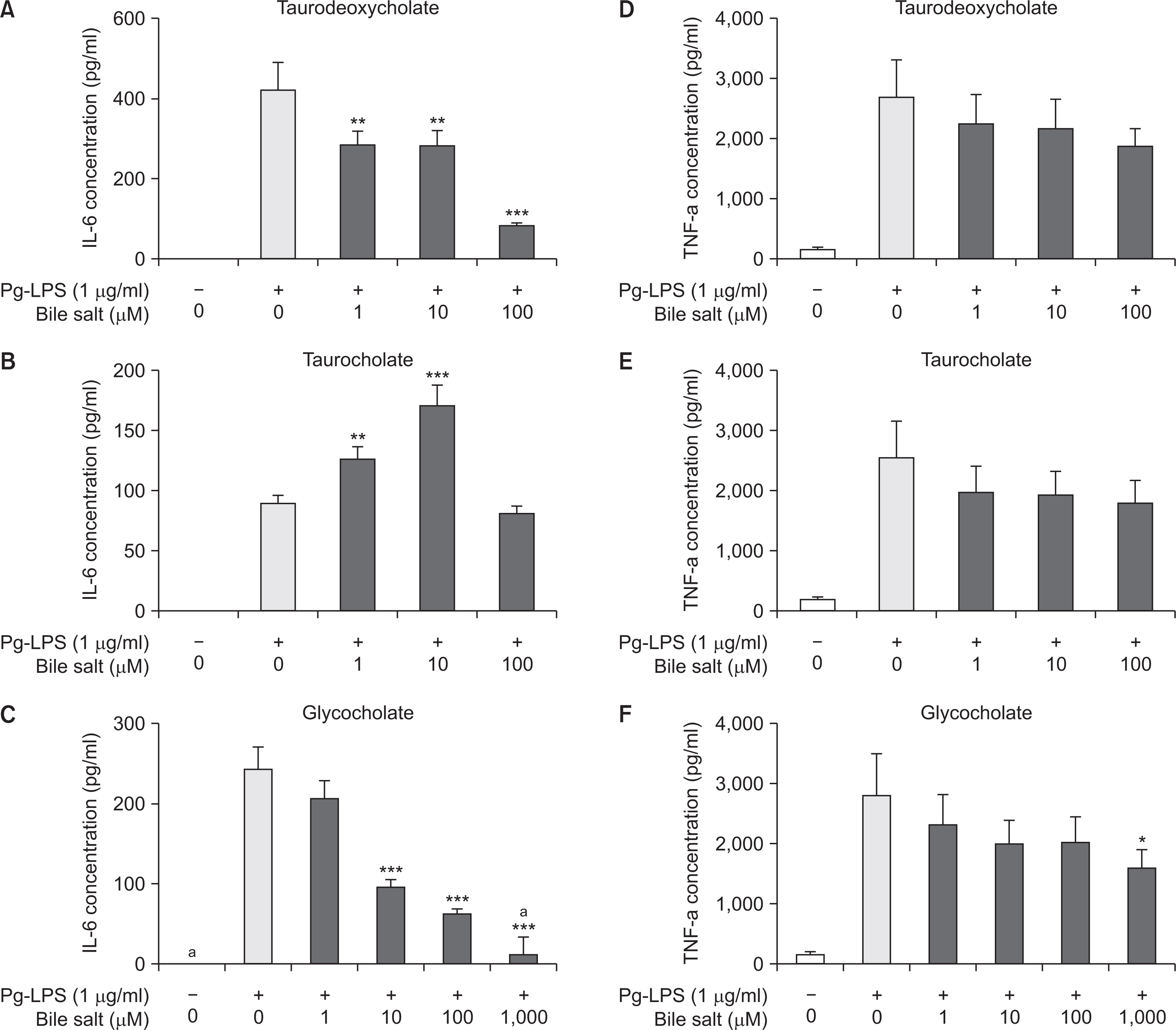
In the cell viability test, taurodeoxycholate and taurocholate did not exhibit any cytotoxic effect on RAW 264.7 cells at concentrations equal to or less than 200 mM, and glycocholate was non-cytotoxic until the maximal concentration (4,000 mM). All the three bile acids exhibited an inhibitory effect on inflammatory response, as the production levels of pro-inflammatory cytokines, including IL-6 and TNF-a, decreased with an increase in the concentration of the three bile acids in a dose-dependent manner. The expression of IL-6 reduced remarkably upon treatment with taurodeoxycholate and glycocholate (P<0.001), while the expression of TNF-a decreased slightly upon treatment with glycocholate (P<0.05). Moreover, only glycocholate at a concentration of 1,000 mM suppressed osteoclast differentiation of RAW 264.7 cells (P<0.001), while taurodeoxycholate and taurocholate did not exhibit an inhibitory effect on osteoclastogenesis.
Conclusions
Here, I showed that all the three bile acids (taurodeoxycholate, taurocholate, and glycocholate) inhibited P. gingivalis LPS-induced inflammatory response, and glycocholate partially suppressed RANKL-mediated osteoclastogenesis in RAW 264.7 cells.
Keyword
Figure
Reference
-
References
1. Yamamoto T, Kita M, Oseko F, Nakamura T, Imanishi J, Kanamura N. 2006; Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodontal Res. 41:554–559. DOI: 10.1111/j.1600-0765.2006.00905.x. PMID: 17076781.
Article2. Kong L, Qi X, Huang S, Chen S, Wu Y, Zhao L. 2015; Theaflavins inhibit pathogenic properties of P. gingivalis and MMPs production in P. gingivalis-stimulated human gingival fibroblasts. Arch Oral Biol. 60:12–22. DOI: 10.1016/j.archoralbio.2014.08.019. PMID: 25244614.
Article3. Lee JH, Kim H, Shim JH, Park J, Lee SK, Park KK, et al. 2018; Platycarya strobilacea leaf extract inhibits tumor necrosis factor-a production and bone loss induced by Porphyromonas gingivalis-derived lipopolysaccharide. Arch Oral Biol. 96:46–51. DOI: 10.1016/j.archoralbio.2018.08.011. PMID: 30172945.4. Lee CT, Huang YW, Zhu L, Weltman R. 2017; Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 62:1–12. DOI: 10.1016/j.jdent.2017.04.011. PMID: 28478213.
Article5. Sanz-Martin I, Doolittle-Hall J, Teles RP, Patel M, Belibasakis GN, Hämmerle CHF, et al. 2017; Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing. J Clin Periodontol. 44:1274–1284. DOI: 10.1111/jcpe.12788. PMID: 28766745. PMCID: PMC7209775.
Article6. Ebersole JL. 2003; Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 31:135–166. DOI: 10.1034/j.1600-0757.2003.03109.x. PMID: 12657000.
Article7. Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. 2007; Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 13 Suppl 4:S3–10. DOI: 10.1111/j.1469-0691.2007.01798.x. PMID: 17716290.
Article8. Andrukhov O, Ulm C, Reischl H, Nguyen PQ, Matejka M, Rausch-Fan X. 2011; Serum cytokine levels in periodontitis patients in relation to the bacterial load. J Periodontol. 82:885–892. DOI: 10.1902/jop.2010.100425. PMID: 21138356.
Article9. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. 2014; Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150. DOI: 10.1016/j.diabres.2014.04.006. PMID: 24798950.
Article10. Nibali L, Tatarakis N, Needleman I, Tu YK, D'Aiuto F, Rizzo M, et al. 2013; Clinical review: Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 98:913–920. DOI: 10.1210/jc.2012-3552. PMID: 23386648.11. Sabharwal A, Gomes-Filho IS, Stellrecht E, Scannapieco FA. 2018; Role of periodontal therapy in management of common complex systemic diseases and conditions: An update. Periodontol 2000. 78:212–226. DOI: 10.1111/prd.12226. PMID: 30198128.
Article12. Papi P, Letizia C, Pilloni A, Petramala L, Saracino V, Rosella D, Pompa G. 2018; Peri-implant diseases and metabolic syndrome components: a systematic review. Eur Rev Med Pharmacol Sci. 22:866–875. DOI: 10.26355/eurrev_201802_14364. PMID: 29509232.13. Ma MM, Li Y, Liu XY, Zhu WW, Ren X, Kong GQ, et al. 2015; Cyanidin-3-O-Glucoside Ameliorates Lipopolysaccharide-Induced Injury Both In Vivo and In Vitro Suppression of NF-κB and MAPK Pathways. Inflammation. 38:1669–1682. DOI: 10.1007/s10753-015-0144-y. PMID: 25752620.
Article14. Doyle CJ, Fitzsimmons TR, Marchant C, Dharmapatni AA, Hirsch R, Bartold PM. 2015; Azithromycin suppresses P. gingivalis LPS-induced pro-inflammatory cytokine and chemokine production by human gingival fibroblasts in vitro. Clin Oral Investig. 19:221–227. DOI: 10.1007/s00784-014-1249-7. PMID: 24806810.
Article15. Panahipour L, Nasserzare S, Amer Z, Brücke F, Stähli A, Kreissl A, et al. 2019; The anti-inflammatory effect of milk and dairy products on periodontal cells: an in vitro approach. Clin Oral Investig. 23:1959–1966. DOI: 10.1007/s00784-018-2642-4. PMID: 30238412.
Article16. Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, et al. 2013; Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17:225–235. DOI: 10.1016/j.cmet.2013.01.003. PMID: 23395169.
Article17. Copple BL, Li T. 2016; Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 104:9–21. DOI: 10.1016/j.phrs.2015.12.007. PMID: 26706784. PMCID: PMC4900180.
Article18. Seong SY, Kang CG. 2007. Korean intellectual property office;Daejeon: Korea Patent Application No. 10-2007-0046579.19. Seong SY, Kim YH. 2014. Korean intellectual property office;Daejeon: Korea Patent Application No. 10-2014-0125691.20. Seong SY, Jang SH, Kim YH, Kim YJ, Jung HE. 2013. Korean intellectual property office;Daejeon: Korea Patent Application No. 10-2013-0101064.21. Yang Y, He J, Suo Y, Lv L, Wang J, Huo C, et al. 2016; Anti-inflammatory effect of taurocholate on TNBS-induced ulcerative colitis in mice. Biomed Pharmacother. 81:424–430. DOI: 10.1016/j.biopha.2016.04.037. PMID: 27261622.
Article22. Choi HJ, Yun JW, Kim YH, Kwon E, Hyon MK, Kim JY, et al. 2019; Evaluation of acute and subacute toxicity of sodium taurodeoxycholate in rats. Drug Chem Toxicol. 1–9. DOI: 10.1080/01480545.2019.1609493. PMID: 31215257.
Article23. Toledo A, Yamaguchi J, Wang JY, Bass BL, Turner DJ, Strauch ED. 2004; Taurodeoxycholate stimulates intestinal cell proliferation and protects against apoptotic cell death through activation of NF-kappaB. Dig Dis Sci. 49:1664–1671. DOI: 10.1023/B:DDAS.0000043383.96077.99. PMID: 15573924.24. Guo C, Qi H, Yu Y, Zhang Q, Su J, Yu D, et al. 2015; The G-Protein-Coupled Bile Acid Receptor Gpbar1 (TGR5) Inhibits Gastric Inflammation Through Antagonizing NF-κB Signaling Pathway. Front Pharmacol. 6:287. DOI: 10.3389/fphar.2015.00287. PMID: 26696888. PMCID: PMC4675858.
Article25. Bian T, Li L, Lyu J, Cui D, Lei L, Yan F. 2016; Human b-defensin 3 suppresses Porphyromonas gingivalis lipopolysaccharide-induced inflammation in RAW 264.7 cells and aortas of ApoE-deficient mice. Peptides. 82:92–100. DOI: 10.1016/j.peptides.2016.06.002. PMID: 27298203.26. Kats A, Norgård M, Wondimu Z, Koro C, Concha Quezada H, Andersson G, et al. 2016; Aminothiazoles inhibit RANKL- and LPS-mediated osteoclastogenesis and PGE2 production in RAW 264.7 cells. J Cell Mol Med. 20:1128–1138. DOI: 10.1111/jcmm.12814. PMID: 26987561. PMCID: PMC4882984.27. Lee JY. 2017. Effects of bismuth oxide, a component of mineral trioxide aggregate, on osteoclast differentiation [master's thesis]. Seoul national university;Seoul:
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nitric oxide production and inducible nitric oxide synthase expression induced by Porphyromonas gingivalis lipopolysaccharide
- The Lipopolysaccharide from Porphyromonas gingivalis Induces Vascular Permeability
- Antibody response of periodontal patients to Porphyromonas gingivalis heat shock protein
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- The relation between metabolic acid and the virulence of porphyromonas gingivalis serotype b