Yonsei Med J.
2019 Apr;60(4):381-388. 10.3349/ymj.2019.60.4.381.
Knockdown of LncRNA H19 Relieves LPS-Induced Damage by Modulating miR-130a in Osteoarthritis
- Affiliations
-
- 1Department of Orthopedics, The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, China. w0309211@sina.com
- 2Department of Orthopedics, Chongqing Dongnan Hospital, Chongqing, China.
- 3Department One of Orthopedics, Dianjiang People's Hospital of Chongqing, Dianjiang County, Chongqing, China.
- KMID: 2450194
- DOI: http://doi.org/10.3349/ymj.2019.60.4.381
Abstract
- PURPOSE
Osteoarthritis (OA) is a commonly occurring illness without a definitive cure, at present. Long non-coding RNAs (lncRNAs) have been widely confirmed to be involved in the modulation of OA progression. This study aimed to investigate the role and mechanism of lncRNA H19 in OA.
MATERIALS AND METHODS
Abundances of H19 and microRNA-130a (miR-130a) in lipopolysaccharide (LPS)-treated C28/I2 cells were measured by reverse-transcription quantitative PCR (RT-qPCR). CCK-8 and flow cytometry analyses were carried out to assess cell viability and apoptosis. Starbase online software was used to predict the putative binding sites between H19 and miR-130a. Luciferase reporter, RNA pull down, and RT-qPCR were performed to analyze the true interaction between H19 and miR-130a.
RESULTS
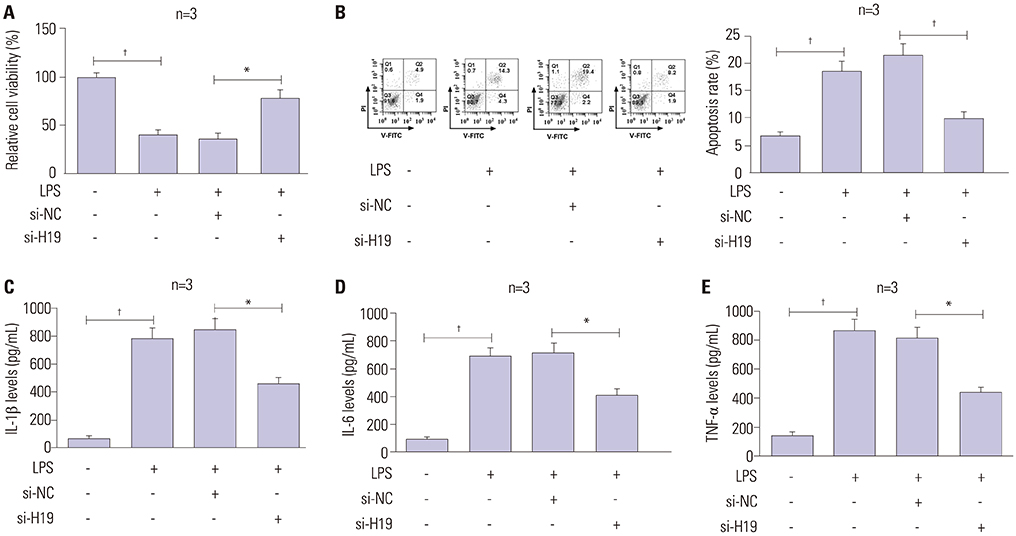
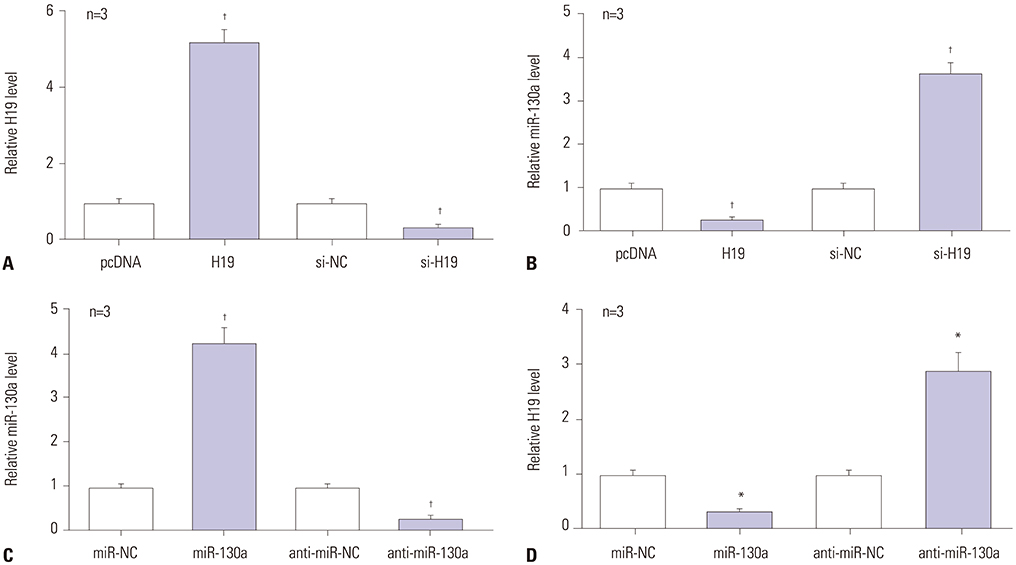
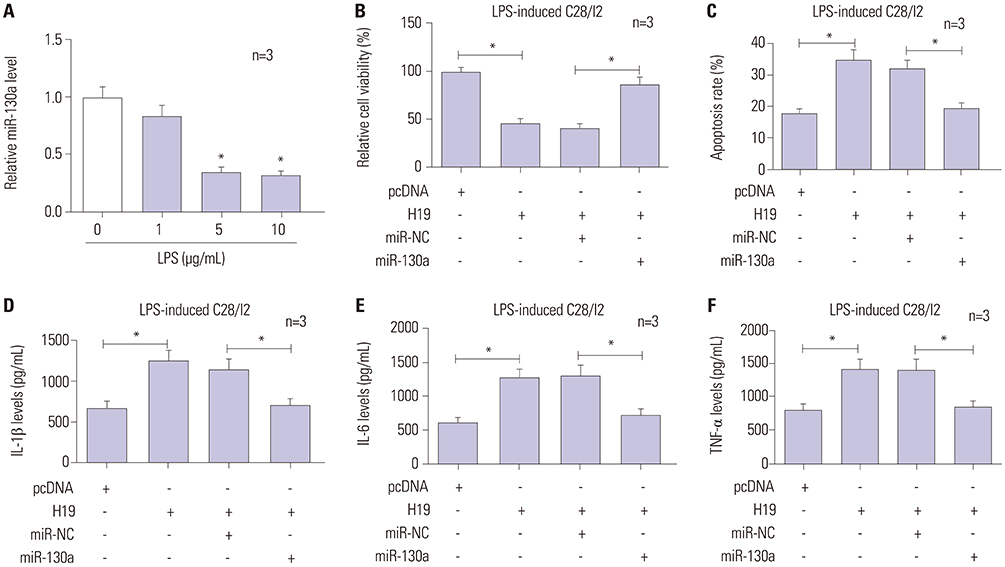
A notably dose-dependent elevation of H19 levels was observed in LPS-treated C28/I2 cells. Knockdown of H19 ameliorated the injury of LPS-induced C28/I2 cells, reflected by induced viability, decreased apoptosis, and reduced inflammatory factor secretions. Moreover, H19 negatively regulated the expression of miR-130a via acting as a molecular sponge for miR-130a. The stimulatory effects of H19 on cell damage were abolished following the restoration of miR-130a.
CONCLUSION
LncRNA H19 aggravated the injury of LPS-induced C28/I2 cells by sponging miR-130a, hinting a novel regulatory mechanism and a potential therapeutic target for OA.
Keyword
MeSH Terms
Figure
Reference
-
1. Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016; 28:8–13.
Article2. Wu C, Tian B, Qu X, Liu F, Tang T, Qin A, et al. MicroRNAs play a role in chondrogenesis and osteoarthritis (review). Int J Mol Med. 2014; 34:13–23.
Article3. Findlay DM, Atkins GJ. Osteoblast-chondrocyte interactions in osteoarthritis. Curr Osteoporos Rep. 2014; 12:127–134.
Article4. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014; 73:1323–1330.
Article5. Chen WK, Yu XH, Yang W, Wang C, He WS, Yan YG, et al. lncRNAs: novel players in intervertebral disc degeneration and osteoarthritis. Cell Prolif. 2017; 50:e12313.
Article6. World report on disability. Eur J Disabil Res. 2011; 5:136.7. Musumeci G, Castrogiovanni P, Trovato FM, Weinberg AM, Al-Wasiyah MK, Alqahtani MH, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015; 16:20560–20575.8. Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015; 16:26035–26054.
Article9. Zamli Z, Adams MA, Tarlton JF, Sharif M. Increased chondrocyte apoptosis is associated with progression of osteoarthritis in spontaneous Guinea pig models of the disease. Int J Mol Sci. 2013; 14:17729–17743.
Article10. Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck LS. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003; 11:681–687.
Article11. Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden is associated with knee osteoarthritis (OA). Osteoarthritis Cartilage. 2016; 24:Suppl 1. S329–S330.
Article12. Zhao C, Wang Y, Jin H, Yu T. Knockdown of microRNA-203 alleviates LPS-induced injury by targeting MCL-1 in C28/I2 chondrocytes. Exp Cell Res. 2017; 359:171–178.
Article13. Marques AC, Ponting CP. Intergenic lncRNAs and the evolution of gene expression. Curr Opin Genet Dev. 2014; 27:48–53.
Article14. Kataoka M, Wang DZ. Non-coding RNAs including miRNAs and lncRNAs in cardiovascular biology and disease. Cells. 2014; 3:883–898.
Article15. Liu Q, Hu X, Zhang X, Dai L, Duan X, Zhou C, et al. The TMSB4 pseudogene lncRNA functions as a competing endogenous RNA to promote cartilage degradation in human osteoarthritis. Mol Ther. 2016; 24:1726–1733.
Article16. Han Y, Ma J, Wang J, Wang L. Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Mol Immunol. 2018; 93:107–114.
Article17. Steck E, Boeuf S, Gabler J, Werth N, Schnatzer P, Diederichs S, et al. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med (Berl). 2012; 90:1185–1195.
Article18. Zheng H, Dong X, Liu N, Xia W, Zhou L, Chen X, et al. Regulation and mechanism of mouse miR-130a/b in metabolism-related inflammation. Int J Biochem Cell Biol. 2016; 74:72–83.
Article19. Li ZC, Han N, Li X, Li G, Liu YZ, Sun GX, et al. Decreased expression of microRNA-130a correlates with TNF-α in the development of osteoarthritis. Int J Clin Exp Pathol. 2015; 8:2555–2564.20. Lee W, Yang EJ, Ku SK, Song KS, Bae JS. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation. 2013; 36:94–102.
Article21. Li K, Dan Z, Nie Y, Hu X, Gesang L, Bianba Z, et al. CD14 knockdown reduces lipopolysaccharide-induced cell viability and expression of inflammation-associated genes in gastric cancer cells in vitro and in nude mouse xenografts. Mol Med Rep. 2015; 12:4332–4339.
Article22. Sun T, Yu J, Han L, Tian S, Xu B, Gong X, et al. Knockdown of long non-coding RNA RP11-445H22.4 alleviates LPS-induced injuries by regulation of MiR-301a in osteoarthritis. Cell Physiol Biochem. 2018; 45:832–843.
Article23. Khorkova O, Hsiao J, Wahlestedt C. Basic biology and therapeutic implications of lncRNA. Adv Drug Deliv Rev. 2015; 87:15–24.
Article24. Quan M, Chen J, Zhang D. Exploring the secrets of long noncoding RNAs. Int J Mol Sci. 2015; 16:5467–5496.
Article25. Li Y, Li S, Luo Y, Liu Y, Yu N. LncRNA PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-488-3p. DNA Cell Biol. 2017; 36:571–580.
Article26. Tay Y, Karreth FA, Pandolfi PP. Aberrant ceRNA activity drives lung cancer. Cell Res. 2014; 24:259–260.
Article27. Xu J, Xu Y. The lncRNA MEG3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis. Cell Biosci. 2017; 7:69.
Article28. Li YF, Li SH, Liu Y, Luo YT. Long noncoding RNA CIR promotes chondrocyte extracellular matrix degradation in osteoarthritis by acting as a sponge for Mir-27b. Cell Physiol Biochem. 2017; 43:602–610.
Article29. Zhang G, Wu Y, Xu D, Yan X. Long noncoding RNA UFC1 promotes proliferation of chondrocyte in osteoarthritis by acting as a sponge for miR-34a. DNA Cell Biol. 2016; 35:691–695.
Article30. He L, Wang HY, Zhang L, Huang L, Li JD, Xiong Y, et al. Prognostic significance of low DICER expression regulated by miR-130a in cervical cancer. Cell Death Dis. 2014; 5:e1205.
Article31. Lee SH, Jung YD, Choi YS, Lee YM. Targeting of RUNX3 by miR-130a and miR-495 cooperatively increases cell proliferation and tumor angiogenesis in gastric cancer cells. Oncotarget. 2015; 6:33269–33278.
Article32. Jin F, Xing J. Circulating miR-126 and miR-130a levels correlate with lower disease risk, disease severity, and reduced inflammatory cytokine levels in acute ischemic stroke patients. Neurol Sci. 2018; 39:1757–1765.
Article33. Song CL, Liu B, Shi YF, Liu N, Yan YY, Zhang JC, et al. MicroRNA-130a alleviates human coronary artery endothelial cell injury and inflammatory responses by targeting PTEN via activating PI3K/Akt/eNOS signaling pathway. Oncotarget. 2016; 7:71922–71936.
Article34. Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000; 43:1916–1926.
Article35. Goldring MB. Immortalization of human articular chondrocytes for generation of stable, differentiated cell lines. Methods Mol Med. 2004; 100:23–36.
Article36. Finger F, Schörle C, Zien A, Gebhard P, Goldring MB, Aigner T. Molecular phenotyping of human chondrocyte cell lines T/C-28a2, T/C-28a4, and C-28/I2. Arthritis Rheum. 2003; 48:3395–3403.
Article37. Loeser RF, Sadiev S, Tan L, Goldring MB. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for α1β1 and α2β1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthritis Cartilage. 2000; 8:96–105.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RETRACTION: Circular RNA-ZFR Inhibited Cell Proliferation and Promoted Apoptosis in Gastric Cancer by Sponging miR-130a/miR-107 and Modulating PTEN
- Circular RNA-ZFR Inhibited Cell Proliferation and Promoted Apoptosis in Gastric Cancer by Sponging miR-130a/miR-107 and Modulating PTEN
- Long non-coding RNA T-cell leukemia/lymphoma 6 serves as a sponge for miR-21 modulating the cell proliferation of retinoblastoma through PTEN
- Long Non-Coding RNA RMRP Contributes to Sepsis-Induced Acute Kidney Injury
- Knockdown of Long Non-Coding RNA NEAT1 Inhibits Proliferation and Invasion and Induces Apoptosis of Osteosarcoma by Inhibiting miR-194 Expression