Yonsei Med J.
2017 Nov;58(6):1092-1100. 10.3349/ymj.2017.58.6.1092.
Knockdown of Long Non-Coding RNA NEAT1 Inhibits Proliferation and Invasion and Induces Apoptosis of Osteosarcoma by Inhibiting miR-194 Expression
- Affiliations
-
- 1Department of Orthopedics, Zhoukou Central Hospital, Zhoukou, China. wangheping112@yeah.net
- 2Department of Surgery, Zhoukou Central Hospital, Zhoukou, China.
- KMID: 2418890
- DOI: http://doi.org/10.3349/ymj.2017.58.6.1092
Abstract
- PURPOSE
Long non-coding RNA (lncRNA) nuclear paraspeckle assembly transcript 1 (NEAT1) has been implicated as an oncogene in the development and progression of osteosarcoma. This study aims to explore the mechanism of NEAT1 in osteosarcoma.
MATERIALS AND METHODS
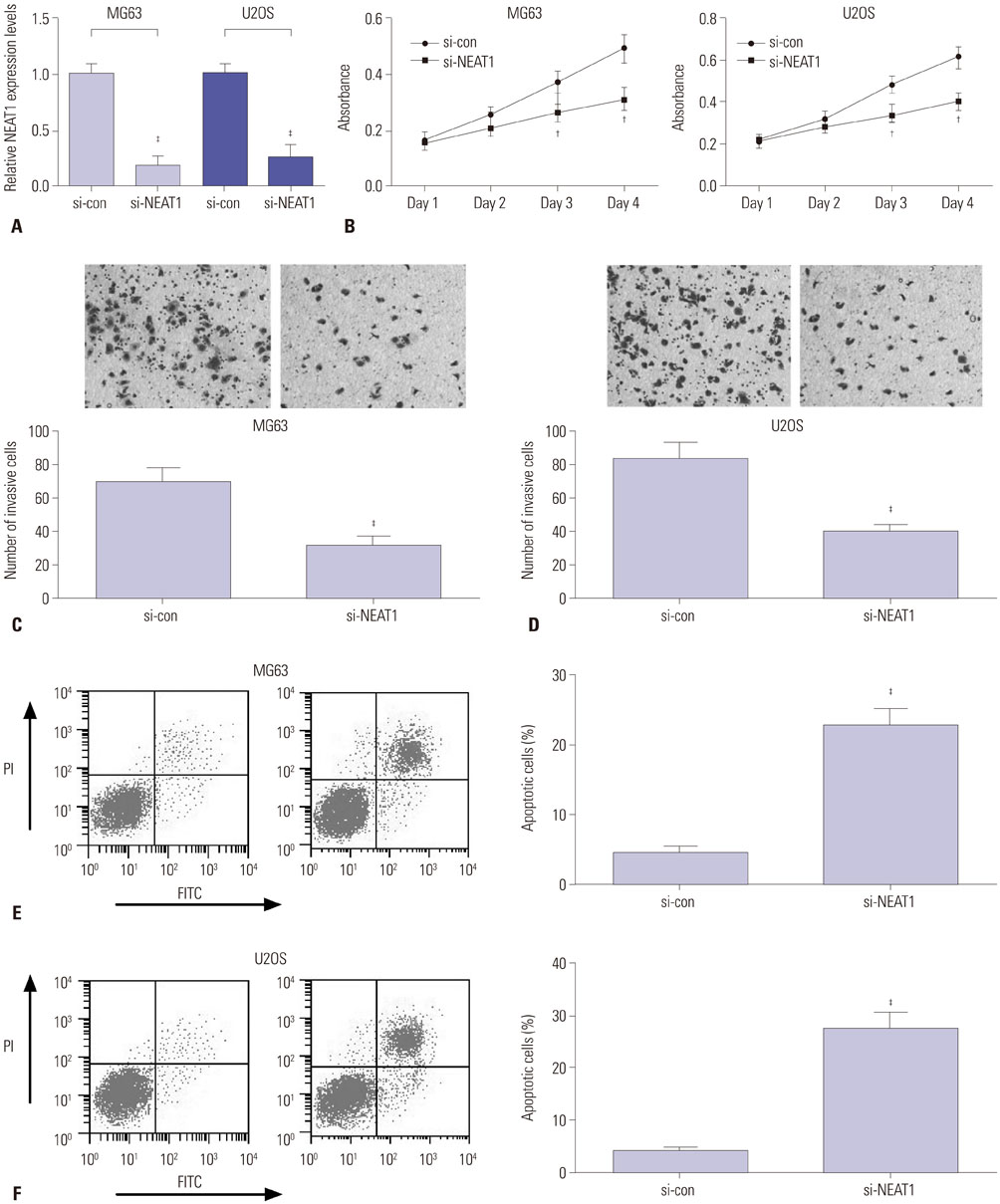
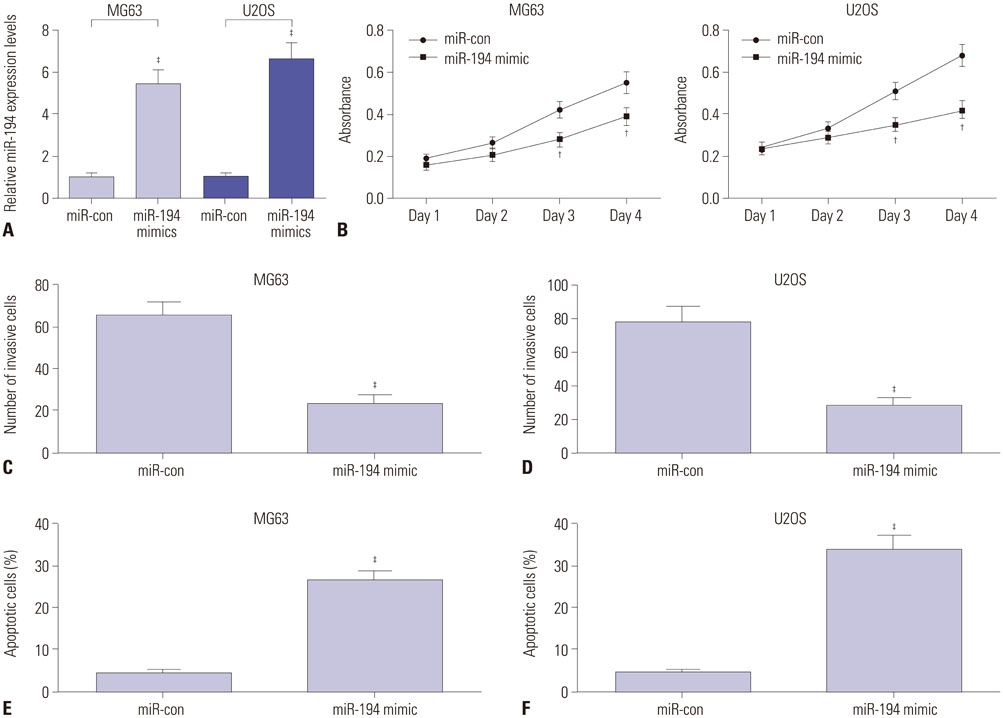
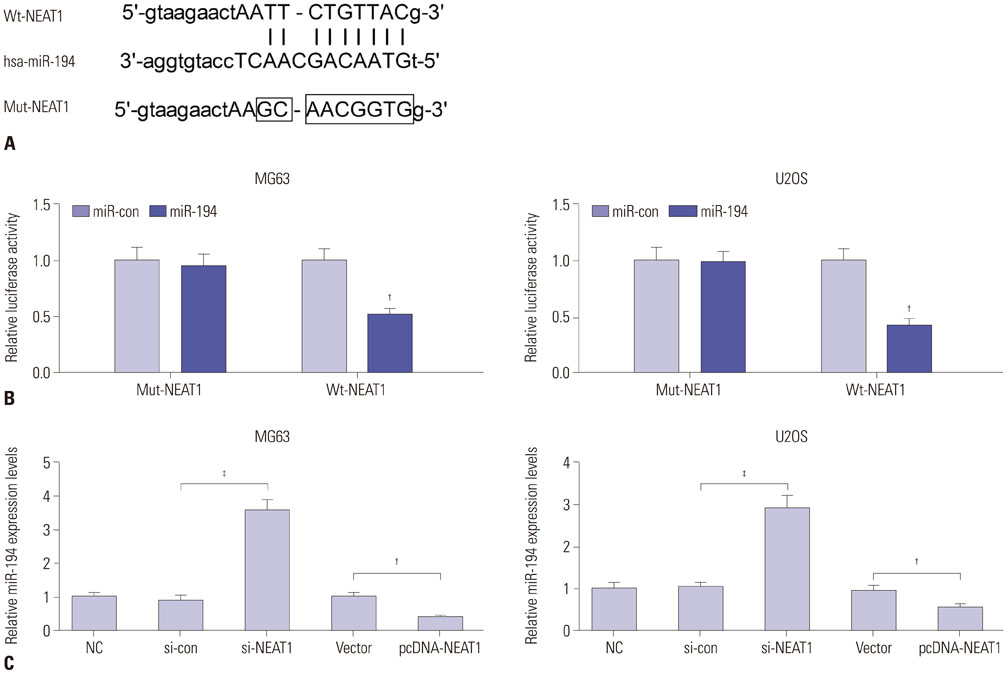
Expressions of NEAT1 and miR-194 in osteosarcoma tissues and cells were detected by quantitative real-time PCR. The effects of NEAT1 knockdown or miR-194 overexpression on cell proliferation, invasion, and apoptosis were determined by 3-[4, 5-dimethylthiazol-2-yl]-2, 5 diphenyl tetrazolium bromide (MTT) assay, transwell invasive assay, and flow cytometry analysis, respectively. Luciferase reporter assay was performed to observe the possible interaction between NEAT1 and miR-194.
RESULTS
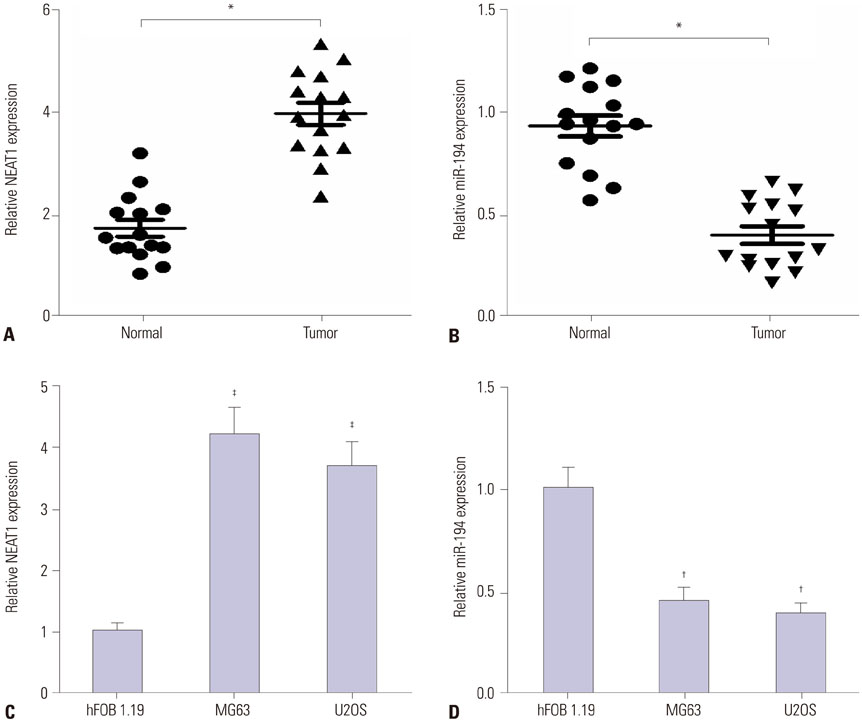
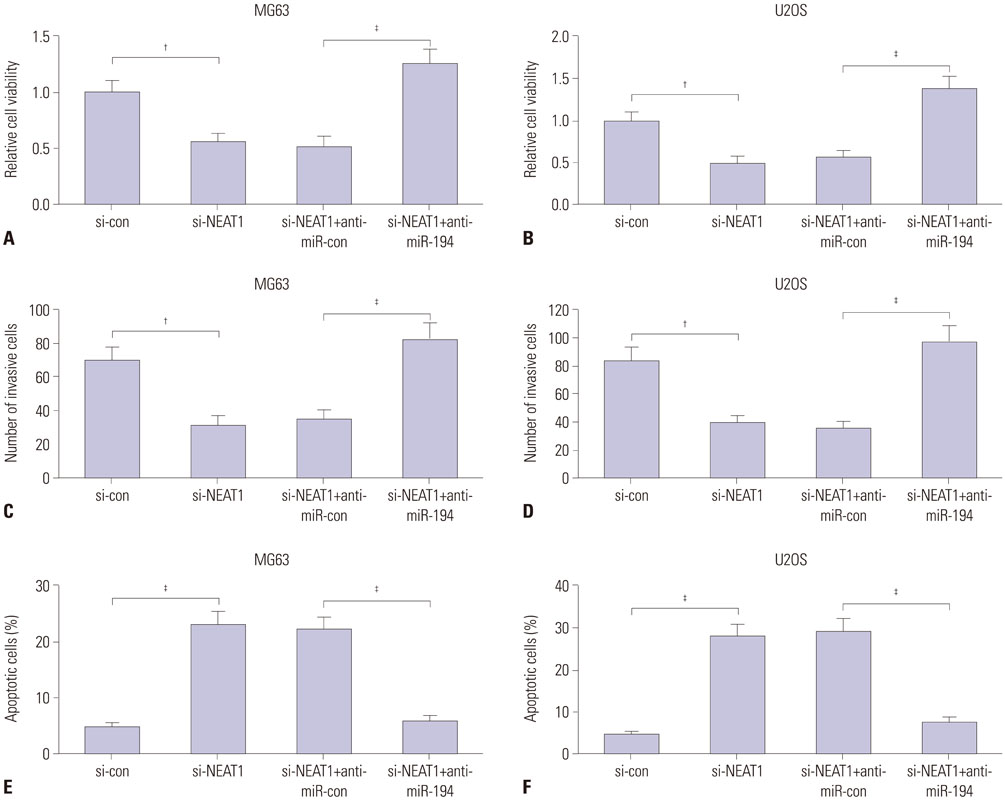
NEAT1 was upregulated and miR-194 was downregulated in osteosarcoma tissues and cells. Knockdown of NEAT1 or overexpression of miR-194 suppressed proliferation and invasion and induced apoptosis of osteosarcoma cells in vitro. Luciferase reporter assay validated that NEAT1 could interact with miR-194 and negatively modulated its expression. Furthermore, inhibition of miR-194 reversed the suppression of proliferation and invasion and the promotion of apoptosis induced by NEAT1 depletion in osteosarcoma cells.
CONCLUSION
Knockdown of NEAT1 suppressed proliferation and invasion and induced apoptosis in osteosarcoma cells by inhibiting miR-194 expression.
Keyword
MeSH Terms
-
Apoptosis/*genetics
Bone Neoplasms/genetics
Cell Proliferation
Disease Progression
Down-Regulation
*Gene Expression Regulation, Neoplastic
Humans
MicroRNAs/*genetics/metabolism
Osteosarcoma/*genetics/pathology
RNA, Long Noncoding/*genetics
Real-Time Polymerase Chain Reaction
Up-Regulation
MicroRNAs
RNA, Long Noncoding
Figure
Reference
-
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009; 152:3–13.
Article2. Stiller CA. International patterns of cancer incidence in adolescents. Cancer Treat Rev. 2007; 33:631–645.
Article3. Yang J, Zhang W. New molecular insights into osteosarcoma targeted therapy. Curr Opin Oncol. 2013; 25:398–406.
Article4. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat Rev. 2014; 40:523–532.
Article5. Chen L, Wang X, Wang H, Li Y, Yan W, Han L, et al. miR-137 is frequently down-regulated in glioblastoma and is a negative regulator of Cox-2. Eur J Cancer. 2012; 48:3104–3111.
Article6. Duan Z, Choy E, Harmon D, Liu X, Susa M, Mankin H, et al. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011; 10:1337–1345.
Article7. Liu LH, Li H, Li JP, Zhong H, Zhang HC, Chen J, et al. miR-125b suppresses the proliferation and migration of osteosarcoma cells through down-regulation of STAT3. Biochem Biophys Res Commun. 2011; 416:31–38.
Article8. Hong Q, Fang J, Pang Y, Zheng J. Prognostic value of the microRNA-29 family in patients with primary osteosarcomas. Med Oncol. 2014; 31:37.
Article9. Meng Z, Fu X, Chen X, Zeng S, Tian Y, Jove R, et al. miR-194 is a marker of hepatic epithelial cells and suppresses metastasis of liver cancer cells in mice. Hepatology. 2010; 52:2148–2157.
Article10. Dong P, Kaneuchi M, Watari H, Hamada J, Sudo S, Ju J, et al. MicroRNA-194 inhibits epithelial to mesenchymal transition of endometrial cancer cells by targeting oncogene BMI-1. Mol Cancer. 2011; 10:99.
Article11. Song Y, Zhao F, Wang Z, Liu Z, Chiang Y, Xu Y, et al. Inverse association between miR-194 expression and tumor invasion in gastric cancer. Ann Surg Oncol. 2012; 19:Suppl 3. S509–S517.
Article12. Han K, Zhao T, Chen X, Bian N, Yang T, Ma Q, et al. microRNA-194 suppresses osteosarcoma cell proliferation and metastasis in vitro and in vivo by targeting CDH2 and IGF1R. Int J Oncol. 2014; 45:1437–1449.
Article13. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009; 10:155–159.
Article14. Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013; 108:2419–2425.
Article15. Li W, Xie P, Ruan WH. Overexpression of lncRNA UCA1 promotes osteosarcoma progression and correlates with poor prognosis. J Bone Oncol. 2016; 5:80–85.
Article16. Cong M, Li J, Jing R, Li Z. Long non-coding RNA tumor suppressor candidate 7 functions as a tumor suppressor and inhibits proliferation in osteosarcoma. Tumour Biol. 2016; 37:9441–9450.
Article17. Yun-Bo F, Xiao-Po L, Xiao-Li L, Guo-Long C, Pei Z, Fa-Ming T. LncRNA TUG1 is upregulated and promotes cell proliferation in osteosarcoma. Open Med (Wars). 2016; 11:163–167.
Article18. Yin Z, Ding H, He E, Chen J, Li M. Overexpression of long non-coding RNA MFI2 promotes cell proliferation and suppresses apoptosis in human osteosarcoma. Oncol Rep. 2016; 36:2033–2040.
Article19. Choudhry H, Albukhari A, Morotti M, Haider S, Moralli D, Smythies J, et al. Tumor hypoxia induces nuclear paraspeckle formation through HIF-2α dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene. 2015; 34:4482–4490.
Article20. Zhao H, Zhao Y, Tao J, Ma C, Zhang J, Xu H, et al. Up-regulated expression of lncRNA NEAT1 promotes progression of osteosarcoma by regulating the activity of Wnt/β-catenin pathway. Int J Clin Exp Pathol. 2016; 9:11466–11472.21. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3:1101–1108.
Article22. Dong Y, Liang G, Yuan B, Yang C, Gao R, Zhou X. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol. 2015; 36:1477–1486.
Article23. Li Z, Zhao L, Wang Q. Overexpression of long non-coding RNA HOTTIP increases chemoresistance of osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J Transl Res. 2016; 8:2385–2393.24. Sun XH, Yang LB, Geng XL, Wang R, Zhang ZC. Increased expression of lncRNA HULC indicates a poor prognosis and promotes cell metastasis in osteosarcoma. Int J Clin Exp Pathol. 2015; 8:2994–3000.25. Zhao H, Hou W, Tao J, Zhao Y, Wan G, Ma C, et al. Upregulation of lncRNA HNF1A-AS1 promotes cell proliferation and metastasis in osteosarcoma through activation of the Wnt/β-catenin signaling pathway. Am J Transl Res. 2016; 8:3503–3512.26. Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009; 33:717–726.
Article27. Jiang P, Wu X, Wang X, Huang W, Feng Q. NEAT1 upregulates EGCG-induced CTR1 to enhance cisplatin sensitivity in lung cancer cells. Oncotarget. 2016; 7:43337–43351.
Article28. Wang P, Wu T, Zhou H, Jin Q, He G, Yu H, et al. Long noncoding RNA NEAT1 promotes laryngeal squamous cell cancer through regulating miR-107/CDK6 pathway. J Exp Clin Cancer Res. 2016; 35:22.
Article29. Chen X, Kong J, Ma Z, Gao S, Feng X. Up regulation of the long noncoding RNA NEAT1 promotes esophageal squamous cell carcinoma cell progression and correlates with poor prognosis. Am J Cancer Res. 2015; 5:2808–2815.30. Stahlhut C, Slack FJ. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Med. 2013; 5:111.
Article31. Zhou Q, Chen F, Zhao J, Li B, Liang Y, Pan W, et al. Long non-coding RNA PVT1 promotes osteosarcoma development by acting as a molecular sponge to regulate miR-195. Oncotarget. 2016; 7:82620–82633.
Article32. Xie CH, Cao YM, Huang Y, Shi QW, Guo JH, Fan ZW, et al. Long non-coding RNA TUG1 contributes to tumorigenesis of human osteosarcoma by sponging miR-9-5p and regulating POU2F1 expression. Tumour Biol. 2016; 37:15031–15041.
Article33. Wu X, Liu T, Fang O, Leach LJ, Hu X, Luo Z. miR-194 suppresses metastasis of non-small cell lung cancer through regulating expression of BMP1 and p27(kip1). Oncogene. 2014; 33:1506–1514.
Article34. Zhang M, Zhuang Q, Cui L. MiR-194 inhibits cell proliferation and invasion via repression of RAP2B in bladder cancer. Biomed Pharmacother. 2016; 80:268–275.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Long Noncoding RNA NEAT1 Targets miR-34a-5p and Drives Nasopharyngeal Carcinoma Progression via Wnt/β-Catenin Signaling
- Long Non-Coding RNA TUG1 Promotes Proliferation and Inhibits Apoptosis of Osteosarcoma Cells by Sponging miR-132-3p and Upregulating SOX4 Expression
- Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/ SOCS1 Axis
- Long Non-Coding RNA LINC00525 Promotes the Stemness and Chemoresistance of Colorectal Cancer by Targeting miR-507/ELK3 Axis
- Long non-coding RNA MEG3 silencing weakens high glucose-induced mesangial cell injury by decreasing LIN28B expression by sponging and sequestering miR-23c