Korean J Physiol Pharmacol.
2019 Nov;23(6):449-458. 10.4196/kjpp.2019.23.6.449.
Long non-coding RNA T-cell leukemia/lymphoma 6 serves as a sponge for miR-21 modulating the cell proliferation of retinoblastoma through PTEN
- Affiliations
-
- 1Department of Science and Education, Changsha Hospital for Maternal & Child Health Care, Changsha 410007, Hunan, China. 411238793@qq.com, jiayinxy2006@126.com
- 2Department of Orthopedics, Shaoyang County People's Hospital, Shaoyang 422100, Hunan, China.
- 3Department of Ophthalmology, The First Affiliated Hospital of Human Normal University/Hunan Provincial People's Hospital, Changsha 410002, Hunan, China.
- 4Health Management Center, Changsha Hospital for Maternal & Child Health Care, Changsha 410007, Hunan, China.
- KMID: 2461037
- DOI: http://doi.org/10.4196/kjpp.2019.23.6.449
Abstract
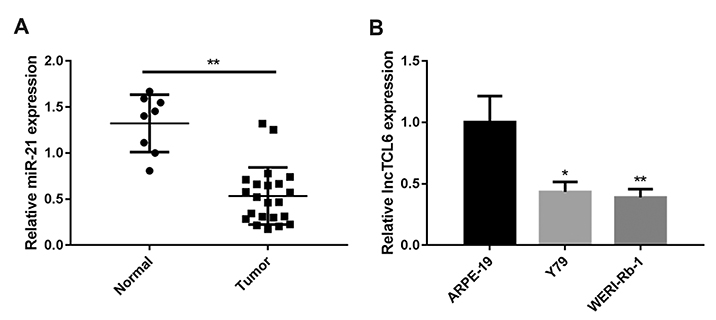
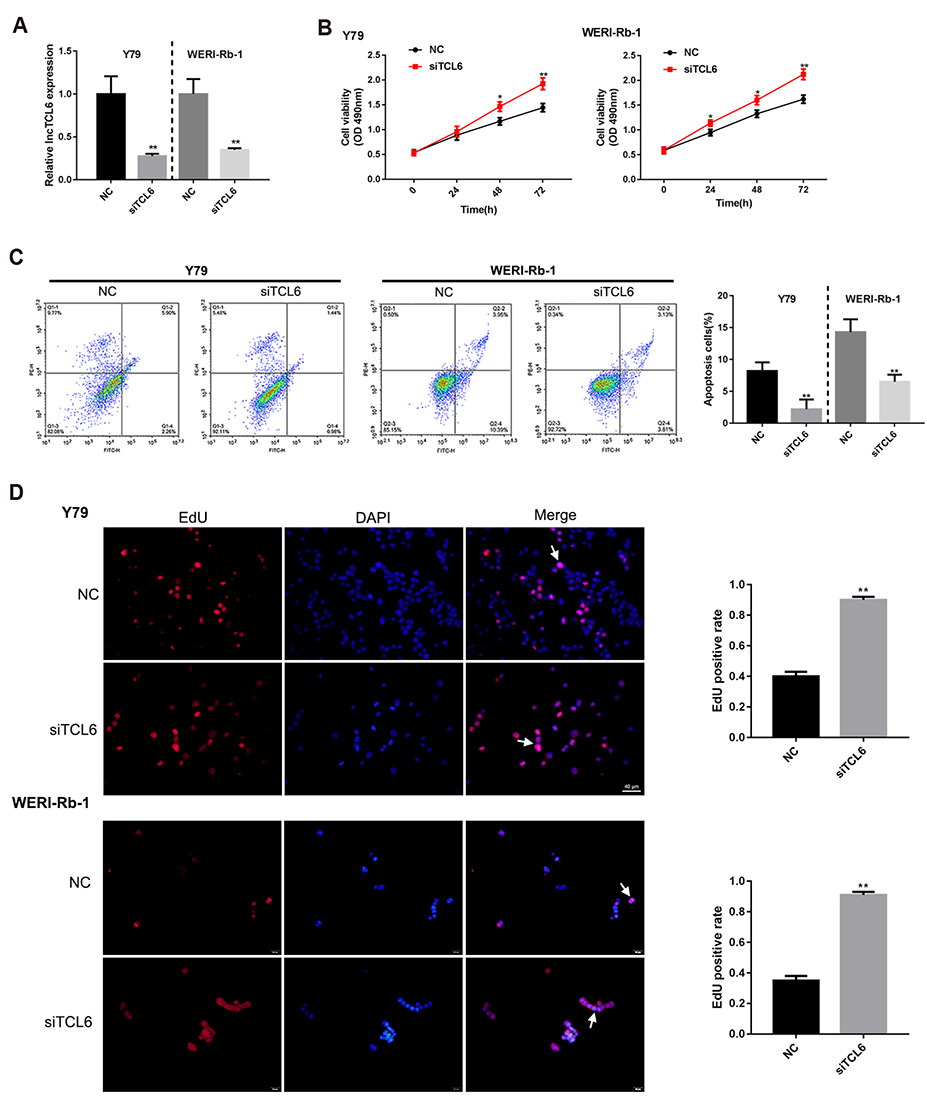
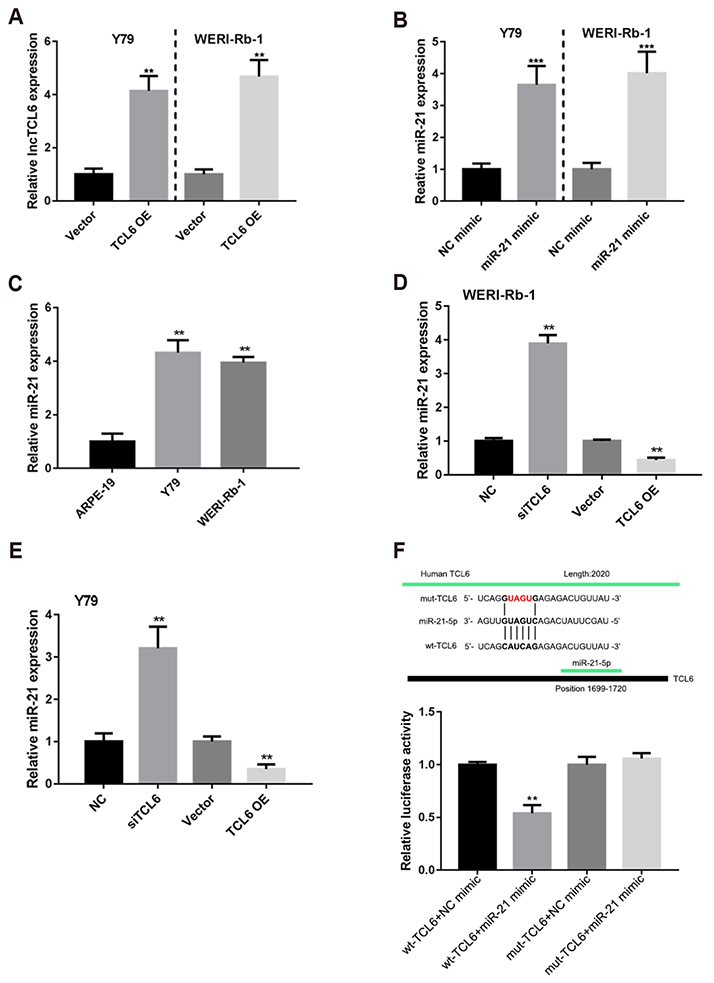
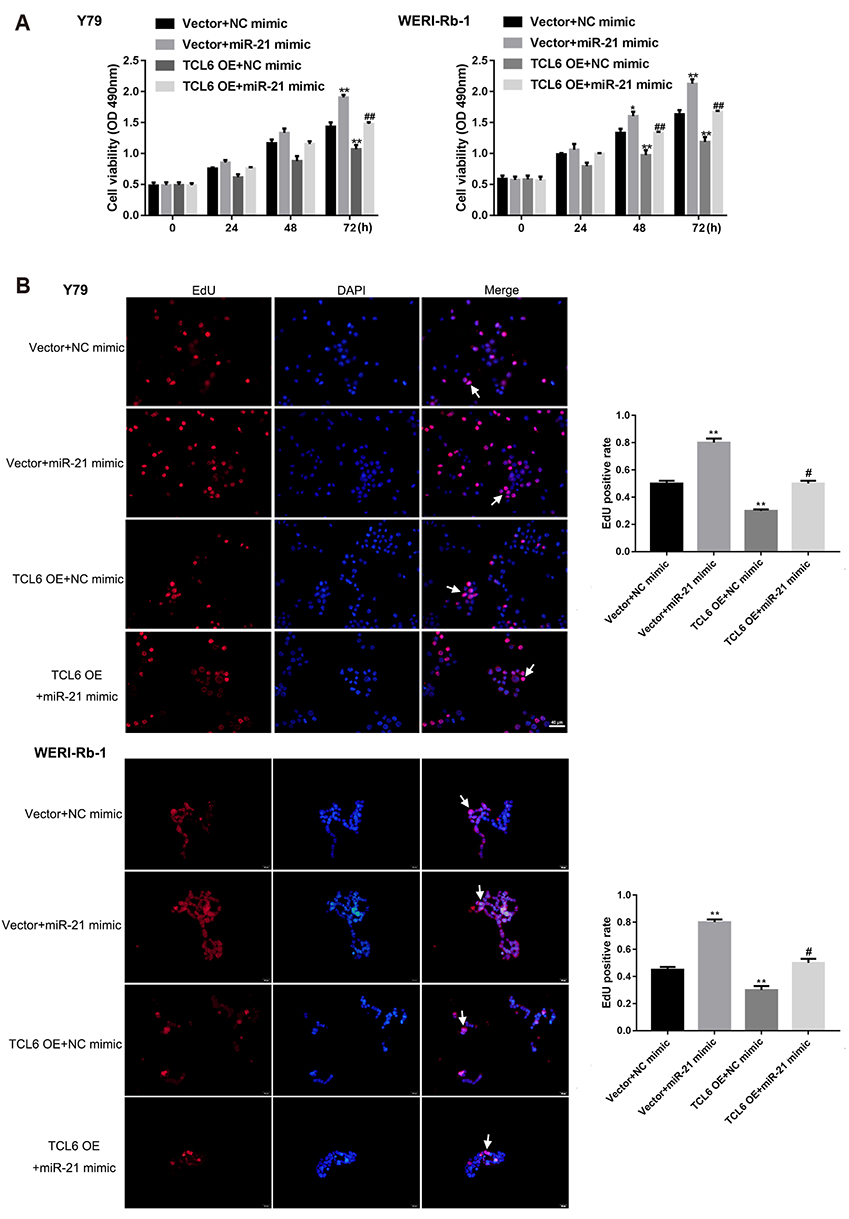
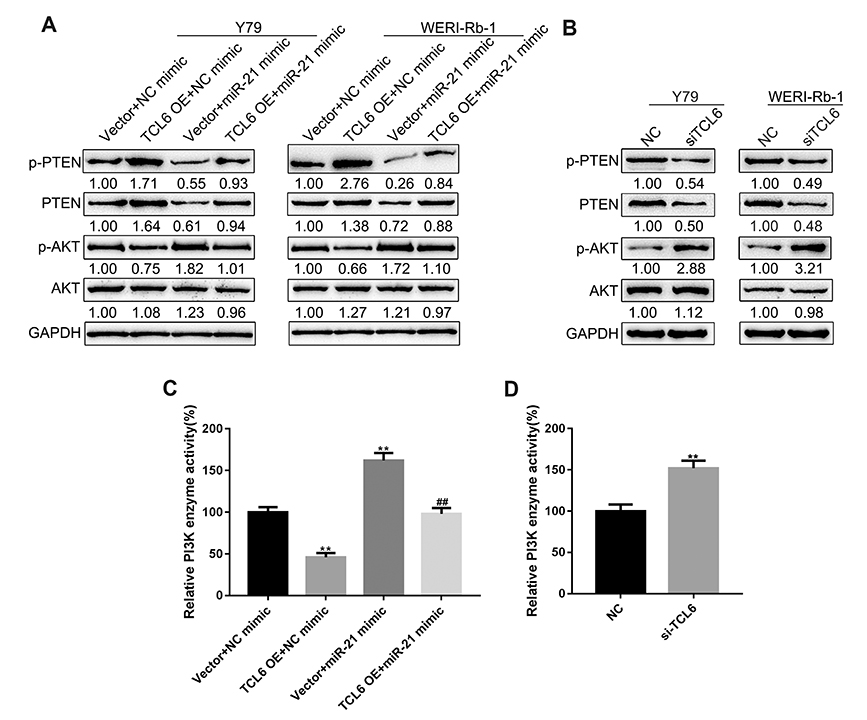
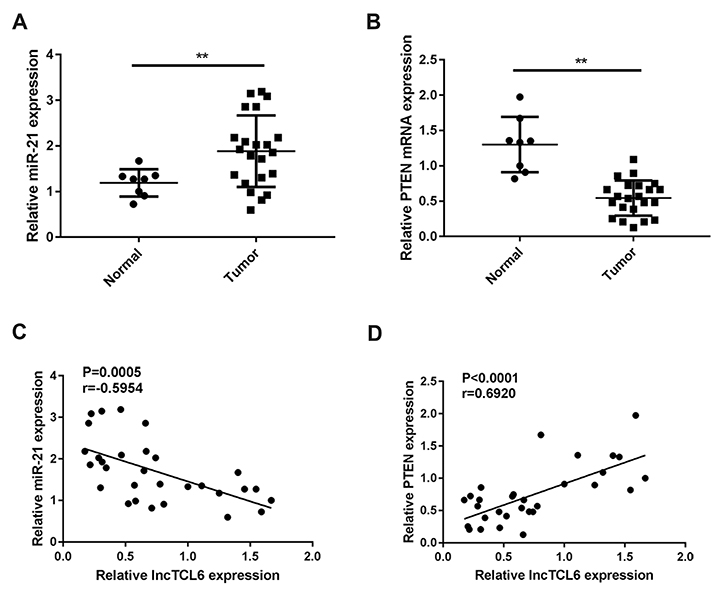
- Retinoblastoma (Rb) is one of the most common eye malignancies occur in childhood. The crucial roles of non-coding RNAs, particularly long non-coding RNAs (lncRNAs) and microRNAs (miRNAs), have been widely reported in Rb progression. In the present study, we found the expression of lncRNA T-cell leukemia/lymphoma 6 (TCL6) was significantly downregulated in Rb tissues and cell lines. Knockdown of lncRNA TCL6 promoted cell proliferation while reduced cell apoptosis in Rb cells. Moreover, lncRNA TCL6 serves as a sponge for miR-21, a previously-reported oncogenic miRNA in Rb, by direct targeting to negatively regulated miR-21 expression, therefore modulating Rb proliferation through miR-21. TCL6 overexpression inhibited Rb cell proliferation while miR-21 overexpression exerted an opposing effect; the effect of TCL6 overexpression was partially attenuated by miR-21 overexpression. PTEN/PI3K/AKT signaling pathway was involved in lncRNA TCL6/miR-21 axis modulating Rb cell proliferation. Taken together, lncRNA TCL6 serves as a tumor suppressor by acting as a sponge for miR-21 to counteract miR-21-mediated PTEN repression.
Keyword
MeSH Terms
Figure
Reference
-
1. Golabchi K, Soleimani-Jelodar R, Aghadoost N, Momeni F, Moridikia A, Nahand JS, Masoudifar A, Razmjoo H, Mirzaei H. MicroRNAs in retinoblastoma: potential diagnostic and therapeutic biomarkers. J Cell Physiol. 2018; 233:3016–3023.
Article2. Pérez-Ramírez C, Cañadas-Garre M, Molina MÁ, Faus-Dáder MJ, Calleja-Hernández MÁ. PTEN and PI3K/AKT in non-small-cell lung cancer. Pharmacogenomics. 2015; 16:1843–1862.
Article3. Chen H, Zhou L, Wu X, Li R, Wen J, Sha J, Wen X. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci (Landmark Ed). 2016; 21:1084–1091.4. Gui F, Hong Z, You Z, Wu H, Zhang Y. MiR-21 inhibitor suppressed the progression of retinoblastoma via the modulation of PTEN/PI3K/AKT pathway. Cell Biol Int. 2016; 40:1294–1302.
Article5. Wei D, Miao Y, Yu L, Wang D, Wang Y. Downregulation of microRNA-198 suppresses cell proliferation and invasion in retinoblastoma by directly targeting PTEN. Mol Med Rep. 2018; 18:595–602.
Article6. Zou WW, Xu SP. Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and Caspase-3 pathways in retinoblastoma. Biomed Pharmacother. 2018; 97:851–863.
Article7. Xie C, Lu H, Nomura A, Hanse EA, Forster CL, Parker JB, Linden MA, Karasch C, Hallstrom TC. Co-deleting Pten with Rb in retinal progenitor cells in mice results in fully penetrant bilateral retinoblastomas. Mol Cancer. 2015; 14:93.
Article8. Su S, Gao J, Wang T, Wang J, Li H, Wang Z. Long non-coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumour Biol. 2015; 36:7205–7211.
Article9. Hao F, Mou Y, Zhang L, Wang S, Yang Y. LncRNA AFAP1-AS1 is a prognostic biomarker and serves as oncogenic role in retinoblastoma. Biosci Rep. 2018; 38:BSR20180384.
Article10. Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006; 103:2257–2261.
Article11. Abtin M, Alivand MR, Khaniani MS, Bastami M, Zaeifizadeh M, Derakhshan SM. Simultaneous downregulation of miR-21 and miR-155 through oleuropein for breast cancer prevention and therapy. J Cell Biochem. 2018; 119:7151–7165.
Article12. Markou A, Zavridou M, Lianidou ES. miRNA-21 as a novel therapeutic target in lung cancer. Lung Cancer (Auckl). 2016; 7:19–27.13. LArki P, Ahadi A, Zare A, Tarighi S, Zaheri M, Souri M, Zali MR, Ghaedi H, Omrani MD. Up-regulation of miR-21, miR-25, miR-93, and miR-106b in gastric cancer. Iran Biomed J. 2018; 22:367–373.
Article14. Shen F, Mo MH, Chen L, An S, Tan X, Fu Y, Rezaei K, Wang Z, Zhang L, Fu SW. MicroRNA-21 down-regulates Rb1 expression by targeting PDCD4 in retinoblastoma. J Cancer. 2014; 5:804–812.
Article15. Yamamura S, Imai-Sumida M, Tanaka Y, Dahiya R. Interaction and cross-talk between non-coding RNAs. Cell Mol Life Sci. 2018; 75:467–484.
Article16. Yang FY, Wang Y, Wu JG, Song SL, Huang G, Xi WM, Tan LL, Wang J, Cao Q. Analysis of long non-coding RNA expression profiles in clear cell renal cell carcinoma. Oncol Lett. 2017; 14:2757–2764.
Article17. Su H, Sun T, Wang H, Shi G, Zhang H, Sun F, Ye D. Decreased TCL6 expression is associated with poor prognosis in patients with clear cell renal cell carcinoma. Oncotarget. 2017; 8:5789–5799.
Article18. Maruyama R, Suzuki H. Long noncoding RNA involvement in cancer. BMB Rep. 2012; 45:604–611.
Article19. Gutschner T, Diederichs S. The hallmarks of cancer: a long noncoding RNA point of view. RNA Biol. 2012; 9:703–719.20. Shang W, Yang Y, Zhang J, Wu Q. Long noncoding RNA BDNF-AS is a potential biomarker and regulates cancer development in human retinoblastoma. Biochem Biophys Res Commun. 2018; 497:1142–1148.
Article21. Zhang A, Shang W, Nie Q, Li T, Li S. Long non-coding RNA H19 suppresses retinoblastoma progression via counteracting miR-17-92 cluster. J Cell Biochem. 2018; 119:3497–3509.
Article22. Paramio JM, Navarro M, Segrelles C, Gómez-Casero E, Jorcano JL. PTEN tumour suppressor is linked to the cell cycle control through the retinoblastoma protein. Oncogene. 1999; 18:7462–7468.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RETRACTION: Circular RNA-ZFR Inhibited Cell Proliferation and Promoted Apoptosis in Gastric Cancer by Sponging miR-130a/miR-107 and Modulating PTEN
- Circular RNA-ZFR Inhibited Cell Proliferation and Promoted Apoptosis in Gastric Cancer by Sponging miR-130a/miR-107 and Modulating PTEN
- LncRNA MEG3 Regulates Imatinib Resistance in Chronic Myeloid Leukemia via Suppressing MicroRNA-21
- AC092127.1-miR-451a-AE binding protein 2 Signaling Facilitates Malignant Properties of Breast Cancer
- Long Non-Coding RNA NORAD Inhibits Breast Cancer Cell Proliferation and Metastasis by Regulating miR-155-5p/ SOCS1 Axis