Int J Stem Cells.
2019 Mar;12(1):125-138. 10.15283/ijsc18091.
Modulation of Renal Parenchyma in Response to Allogeneic Adipose-Derived Mesenchymal Stem Cells Transplantation in Acute Kidney Injury
- Affiliations
-
- 1Stem Cells Research Laboratory (SCRL), Karachi, Pakistan. sumreenbegum@gmail.com
- 2Clinical Laboratory Sciences (CLS), Karachi, Pakistan.
- 3Department of Urology, Sindh Institute of Urology and Transplantation (SIUT), Karachi, Pakistan.
- KMID: 2447231
- DOI: http://doi.org/10.15283/ijsc18091
Abstract
- BACKGROUND AND OBJECTIVES
In regenerative medicine, mesenchymal stem cells derived from adipose tissues (Ad-MSCs) are a very attractive target to treat many diseases. In relation to nephrology, the aim of the current study is to investigate the effects of Ad-MSCs for the amelioration of acute kidney injury and to explore the mechanism of renal parenchymal changes in response to allogeneic transplantation of Ad-MSCs.
METHODS AND RESULTS
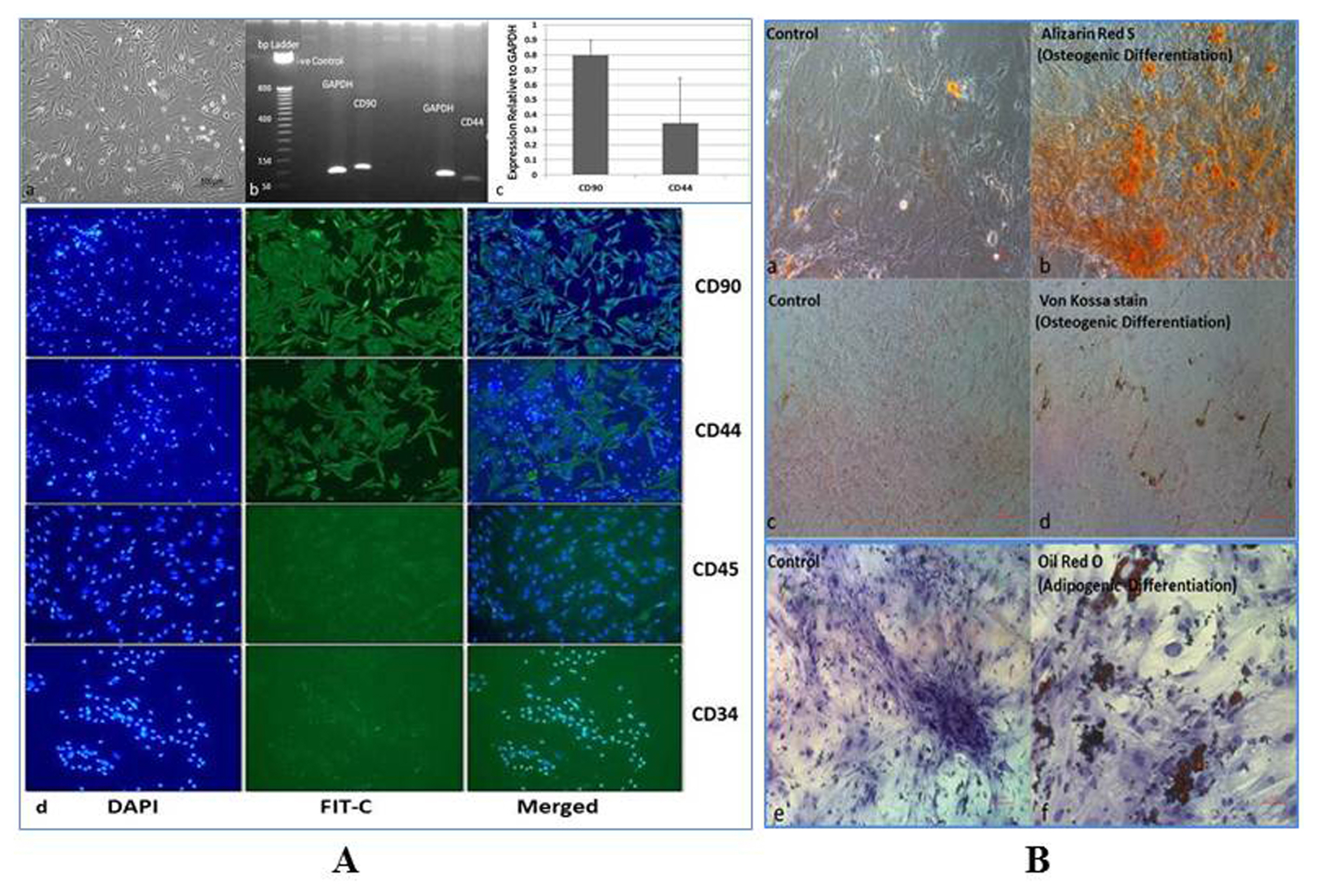
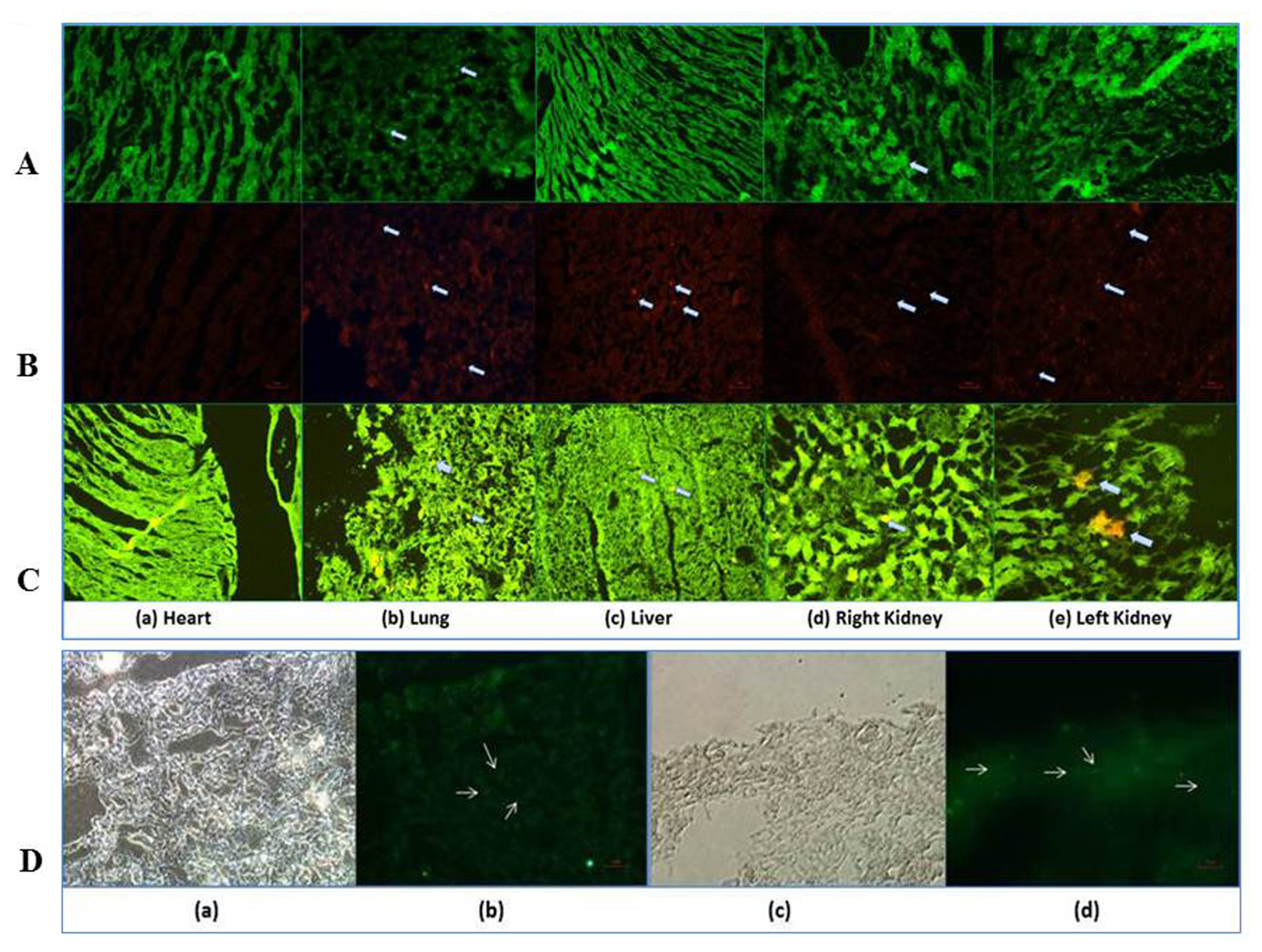
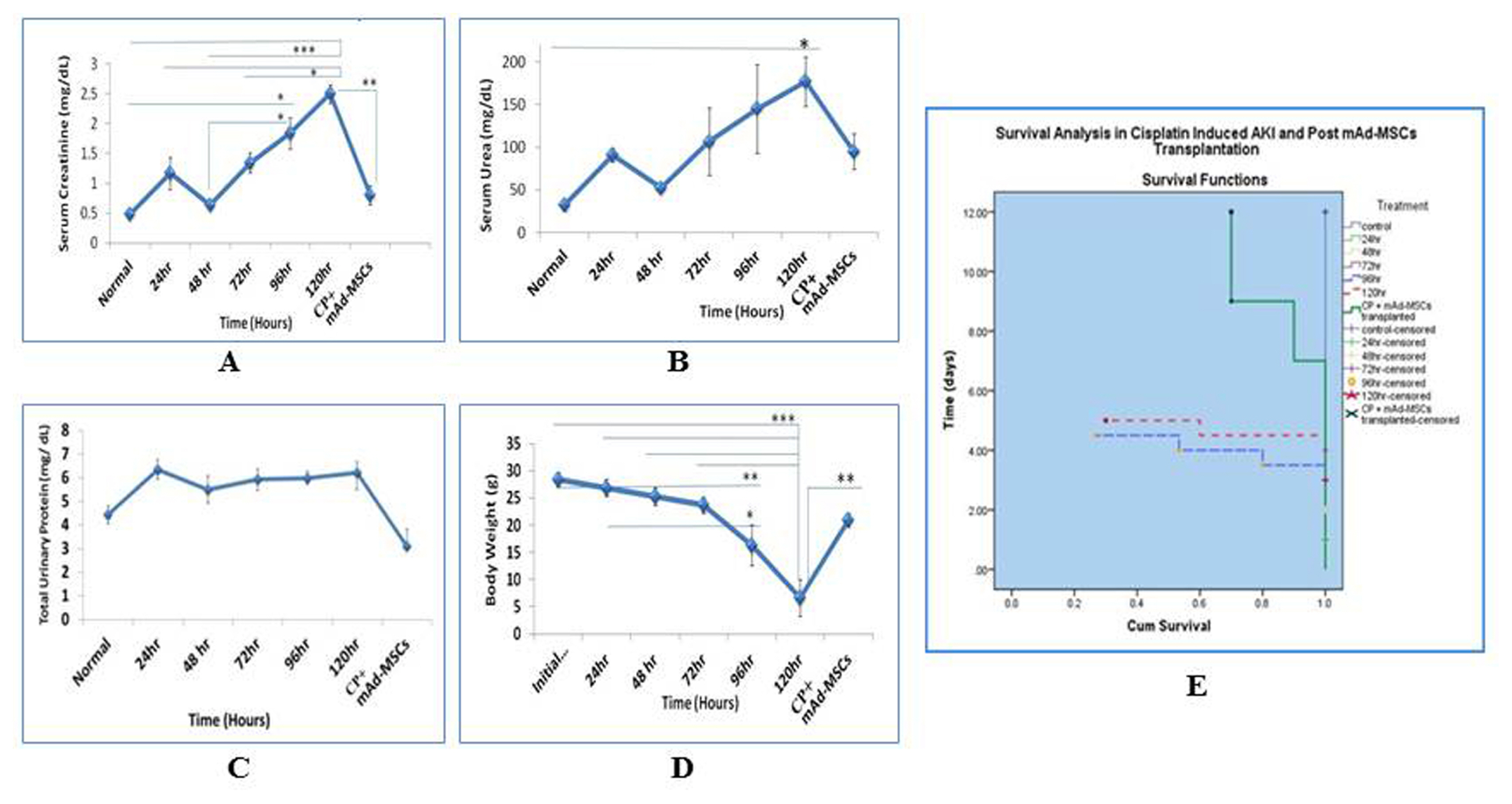
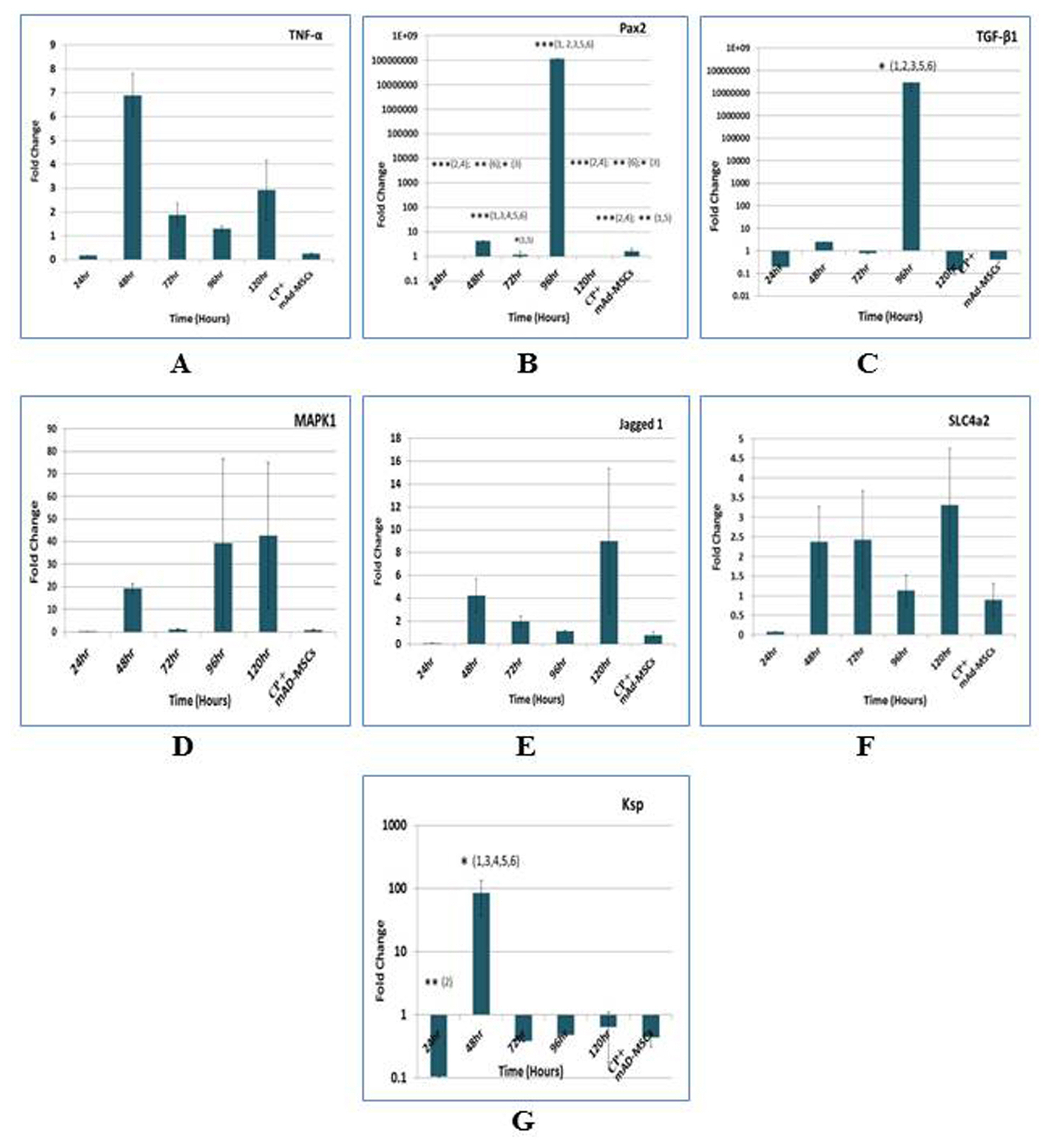
The nephrotoxicity was induced by cisplatin (CP) in balb/c mice according to RIFLE Class and AKIN Stage 3. PCR, qRT-PCR and fluorescent labeled cells infusion, histopathology, immunohistochemistry, functional analyses were used for genes and proteins expressions data acquisition respectively. We demonstrated that single intravenous infusion of 2.5×107/kg mAd-MSCs in mice pre-injected with CP recruited to the kidney, restored the renal structure, and function, which resulted in progressive survival of mice. The renal tissue morphology was recovered in terms of diminished necrosis or epithelial cells damage, protein casts formation, infiltration of inflammatory cells, tubular dilatation, and restoration of brush border protein; Megalin and decreased Kim-1 expressions in mAd-MSCs transplanted mice. Significant reduction in serum creatinine with slashed urea and urinary protein levels were observed. Anti-BrdU staining displayed enhanced tubular cells proliferation. Predominantly, downgrade expressions of TNF-α and TGF-β1 were observed post seven days in mAd-MSCs transplanted mice.
CONCLUSIONS
Ad-MSCs exerts pro-proliferative, anti-inflammatory, and anti-fibrotic effects. Ad-MSCs transplantation without any chemical or genetic manipulation can provide the evidence of therapeutic strategy for the origin of regeneration and overall an improved survival of the system in functionally deprived failed kidneys.
Keyword
MeSH Terms
-
Acute Kidney Injury*
Animals
Cisplatin
Creatinine
Dilatation
Epithelial Cells
Immunohistochemistry
Infusions, Intravenous
Kidney
Low Density Lipoprotein Receptor-Related Protein-2
Mesenchymal Stromal Cells*
Mice
Microvilli
Necrosis
Nephrology
Polymerase Chain Reaction
Regeneration
Regenerative Medicine
Transplantation, Homologous
Urea
Cisplatin
Creatinine
Low Density Lipoprotein Receptor-Related Protein-2
Urea
Figure
Reference
-
References
1. Kanbay M, Covic A. Bone morphogenic protein-7: a new prognostic marker for acute kidney injury? NDT Plus. 2010; 3:106–107. DOI: 10.1093/ndtplus/sfp165. PMID: 25949422. PMCID: 4421545.
Article2. Yao W, Hu Q, Ma Y, Xiong W, Wu T, Cao J, Wu D. Human adipose-derived mesenchymal stem cells repair cisplatin-induced acute kidney injury through antiapoptotic pathways. Exp Ther Med. 2015; 10:468–476. DOI: 10.3892/etm.2015.2505. PMID: 26622339. PMCID: 4509364.
Article3. Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004; 15:1794–1804. DOI: 10.1097/01.ASN.0000128974.07460.34. PMID: 15213267.
Article4. Barnes CJ, Distaso CT, Spitz KM, Verdun VA, Haramati A. Comparison of stem cell therapies for acute kidney injury. Am J Stem Cells. 2016; 5:1–10. PMID: 27335697. PMCID: 4913292.5. Patschan D, Buschmann I, Ritter O, Kribben A. Cell-based therapies in Acute Kidney Injury (AKI). Kidney Blood Press Res. 2018; 43:673–681. DOI: 10.1159/000489624. PMID: 29734169.
Article6. Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011; 7:201–208. DOI: 10.1038/nrneph.2011.14. PMID: 21364520.
Article7. Mohsenin V. Practical approach to detection and management of acute kidney injury in critically ill patient. J Intensive Care. 2017; 5:57. DOI: 10.1186/s40560-017-0251-y. PMID: 28932401. PMCID: 5603084.
Article8. Luna AC, Madeira ME, Conceição TO, Moreira JA, Laiso RA, Maria DA. Characterization of adipose-derived stem cells of anatomical region from mice. BMC Res Notes. 2014; 7:552. DOI: 10.1186/1756-0500-7-552. PMID: 25138545. PMCID: 4156637.
Article9. Večerić-Haler Ž, Erman A, Cerar A, Motaln H, Kološa K, Lah Turnšek T, Sodin Šemrl S, Lakota K, Mrak-Poljšak K, Škrajnar Š, Kranjc S, Arnol M, Perše M. Improved protective effect of umbilical cord stem cell transplantation on cisplatin-induced kidney injury in mice pretreated with antithymocyte globulin. Stem Cells Int. 2016; 2016:3585362. DOI: 10.1155/2016/3585362. PMID: 26880955. PMCID: 4736416.
Article10. Lindoso RS, Verdoorn KS, Einicker-Lamas M. Renal recovery after injury: the role of Pax-2. Nephrol Dial Transplant. 2009; 24:2628–2633. DOI: 10.1093/ndt/gfp307. PMID: 19556301.
Article11. Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014; 2014:967826. DOI: 10.1155/2014/967826. PMID: 25165721. PMCID: 4140112.
Article12. Cheng K, Rai P, Plagov A, Lan X, Kumar D, Salhan D, Rehman S, Malhotra A, Bhargava K, Palestro CJ, Gupta S, Singhal PC. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol. 2013; 94:466–473. DOI: 10.1016/j.yexmp.2013.03.002. PMID: 23534987. PMCID: 3647016.
Article13. Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol. 2002; 13:858–865. PMID: 11912244.
Article14. Christensen EI, Verroust PJ. Megalin and cubilin, role in proximal tubule function and during development. Pediatr Nephrol. 2002; 17:993–999. DOI: 10.1007/s00467-002-0956-5. PMID: 12478347.
Article15. Gekle M, Knaus P, Nielsen R, Mildenberger S, Freudinger R, Wohlfarth V, Sauvant C, Christensen EI. Transforming growth factor-beta1 reduces megalin- and cubilin-mediated endocytosis of albumin in proximal-tubule-derived opossum kidney cells. J Physiol. 2003; 552:471–481. DOI: 10.1113/jphysiol.2003.048074. PMID: 14561830. PMCID: 2343374.
Article16. Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004; 286:F552–563. DOI: 10.1152/ajprenal.00285.2002. PMID: 14600030.
Article17. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006; 290:F517–529. DOI: 10.1152/ajprenal.00291.2005. PMID: 16174863.
Article18. Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009; 24:3265–3268. DOI: 10.1093/ndt/gfp010. PMID: 19318357.
Article19. Ghaly EN, Gergis SW, Aziz JN, Yassa HD, Hassan HA. Role of mesenchymal stem cell therapy in cisplatin induced nephrotoxicity in adult albino rats: ultrastructural & biochemical study. Acta Medica Int. 2014; 1:57–66. DOI: 10.5530/ami.2014.2.3.
Article20. Zhang JB, Wang XQ, Lu GL, Huang HS, Xu SY. Adiposederived mesenchymal stem cells therapy for acute kidney injury induced by ischemia-reperfusion in a rat model. Clin Exp Pharmacol Physiol. 2017; 44:1232–1240. DOI: 10.1111/1440-1681.12811. PMID: 28688148.
Article21. Schubert R, Sann J, Frueh JT, Ullrich E, Geiger H, Baer PC. Tracking of adipose-derived mesenchymal stromal/stem cells in a model of cisplatin-induced acute kidney injury: comparison of bioluminescence imaging versus qRT-PCR. Int J Mol Sci. 2018; 19. pii: E2564. DOI: 10.3390/ijms19092564. PMID: 30158455. PMCID: 6165020.
Article22. Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara K, Hirakata H, Iida M. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003; 63:72–82. DOI: 10.1046/j.1523-1755.2003.00709.x. PMID: 12472770.
Article23. Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005; 289:F166–174. DOI: 10.1152/ajprenal.00401.2004. PMID: 15701814.
Article24. Luo J, Tsuji T, Yasuda H, Sun Y, Fujigaki Y, Hishida A. The molecular mechanisms of the attenuation of cisplatin-induced acute renal failure by N-acetylcysteine in rats. Nephrol Dial Transplant. 2008; 23:2198–2205. DOI: 10.1093/ndt/gfn090. PMID: 18385389.
Article25. Francescato HD, Costa RS, Silva CG, Coimbra TM. Treatment with a p38 MAPK inhibitor attenuates cisplatin nephrotoxicity starting after the beginning of renal damage. Life Sci. 2009; 84:590–597. DOI: 10.1016/j.lfs.2009.02.004. PMID: 26324989.
Article26. Malik S, Suchal K, Bhatia J, Gamad N, Dinda AK, Gupta YK, Arya DS. Molecular mechanisms underlying attenuation of cisplatin-induced acute kidney injury by epicate-chin gallate. Lab Invest. 2016; 96:853–861. DOI: 10.1038/labinvest.2016.60. PMID: 27239733.
Article27. Imgrund M, Gröne E, Gröne HJ, Kretzler M, Holzman L, Schlöndorff D, Rothenpieler UW. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int. 1999; 56:1423–1431. DOI: 10.1046/j.1523-1755.1999.00663.x. PMID: 10504494.
Article28. Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011; 108:9226–9231. DOI: 10.1073/pnas.1100629108. PMID: 21576461. PMCID: 3107336.
Article29. Jiang YS, Jiang T, Huang B, Chen PS, Ouyang J. Epithelial-mesenchymal transition of renal tubules: divergent processes of repairing in acute or chronic injury? Med Hypotheses. 2013; 81:73–75. DOI: 10.1016/j.mehy.2013.03.020. PMID: 23601763.
Article30. Kobayashi T, Terada Y, Kuwana H, Tanaka H, Okado T, Kuwahara M, Tohda S, Sakano S, Sasaki S. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int. 2008; 73:1240–1250. DOI: 10.1038/ki.2008.74. PMID: 18418349.
Article31. Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010; 120:4040–4054. DOI: 10.1172/JCI43025. PMID: 20978353. PMCID: 2964979.
Article32. Nyhan KC, Faherty N, Murray G, Cooey LB, Godson C, Crean JK, Brazil DP. Jagged/Notch signalling is required for a subset of TGFβ1 responses in human kidney epithelial cells. Biochim Biophys Acta. 2010; 1803:1386–1395. DOI: 10.1016/j.bbamcr.2010.09.001. PMID: 20833210.
Article33. Djudjaj S, Chatziantoniou C, Raffetseder U, Guerrot D, Dussaule JC, Boor P, Kerroch M, Hanssen L, Brandt S, Dittrich A, Ostendorf T, Floege J, Zhu C, Lindenmeyer M, Cohen CD, Mertens PR. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol. 2012; 228:286–299. DOI: 10.1002/path.4076. PMID: 22806125.
Article34. Wang Z, Schultheis PJ, Shull GE. Three N-terminal variants of the AE2 Cl-/HCO3− exchanger are encoded by mRNAs transcribed from alternative promoters. J Biol Chem. 1996; 271:7835–7843. DOI: 10.1074/jbc.271.13.7835. PMID: 8631828.
Article35. Medina JF, Lecanda J, Acín A, Ciesielczyk P, Prieto J. Tissue-specific N-terminal isoforms from overlapping alternate promoters of the human AE2 anion exchanger gene. Biochem Biophys Res Commun. 2000; 267:228–235. DOI: 10.1006/bbrc.1999.1951. PMID: 10623603.
Article36. Alper SL, Darman RB, Chernova MN, Dahl NK. The AE gene family of Cl/HCO3− exchangers. J Nephrol. 2002; 15(Suppl 5):S41–53.37. Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO 3 - transporters. Pflugers Arch. 2004; 447:495–509. DOI: 10.1007/s00424-003-1180-2. PMID: 14722772.38. Rodríguez-Soriano J. New insights into the pathogenesis of renal tubular acidosis--from functional to molecular studies. Pediatr Nephrol. 2000; 14:1121–1136. DOI: 10.1007/s004670000407. PMID: 11045400.
Article39. Stewart AK, Kurschat CE, Vaughan-Jones RD, Alper SL. Putative re-entrant loop 1 of AE2 transmembrane domain has a major role in acute regulation of anion exchange by pH. J Biol Chem. 2009; 284:6126–6139. DOI: 10.1074/jbc.M802051200. PMID: 19103596. PMCID: 2649077.
Article40. Jiang J, Dean D, Burghardt RC, Parrish AR. Disruption of cadherin/catenin expression, localization, and interactions during HgCl2-induced nephrotoxicity. Toxicol Sci. 2004; 80:170–182. DOI: 10.1093/toxsci/kfh143. PMID: 15084754.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs
- Rapid deterioration of preexisting renal insufficiency after autologous mesenchymal stem cell therapy
- The Role of Adipose Tissue-Derived Stem Cells in Ischemic Acute Renal Injury in Rats
- Adipose-derived stem cells: characterization and clinical application
- Therapeutic potential of adipose tissue-derived stem cells for liver failure according to the transplantation routes