J Korean Surg Soc.
2011 Sep;81(3):176-186. 10.4174/jkss.2011.81.3.176.
Therapeutic potential of adipose tissue-derived stem cells for liver failure according to the transplantation routes
- Affiliations
-
- 1Department of Surgery, Daejeon St. Mary's Hospital, The Catholic University of Korea School of Medicine, Daejeon, Korea.
- 2Clinical Research Institute, Daejeon St. Mary's Hospital, The Catholic University of Korea School of Medicine, Daejeon, Korea.
- 3Department of Pathology, Daejeon St. Mary's Hospital, The Catholic University of Korea School of Medicine, Daejeon, Korea.
- 4Department of Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea School of Medicine, Seoul, Korea. kimdg@catholic.ac.kr
- KMID: 2212195
- DOI: http://doi.org/10.4174/jkss.2011.81.3.176
Abstract
- PURPOSE
Even though adipose tissue-derived stem cells (ADSCs) have been spotlighted as a possible alternative for liver transplantation in an experimental setting, the mechanism by which ADSCs improve liver dysfunction remains poorly characterized. The objective of this study was to evaluate the therapeutic ability of undifferentiated ADSCs, and find a few clues on how ADSCs alleviate liver damage by comparing the transplantation routes.
METHODS
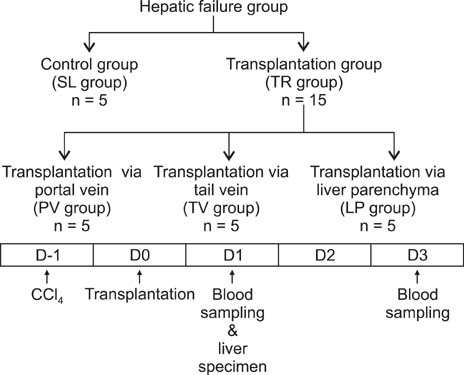
In vitro generated human ADSCs were checked for surface markers and stage-specific genes for characterization. Afterwards, they were transplanted into C57BL/6 mice with CCl4-induced liver injury. The transplantations were made via tail vein, portal vein, and direct liver parenchymal injection. At 1 and 3 post-transplantation days, serum biochemical parameters and/or liver specimens were evaluated.
RESULTS
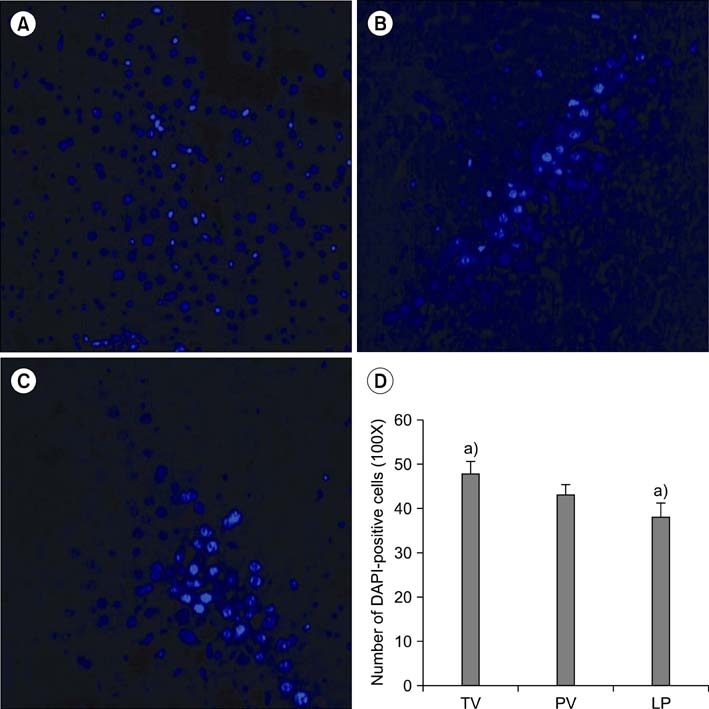
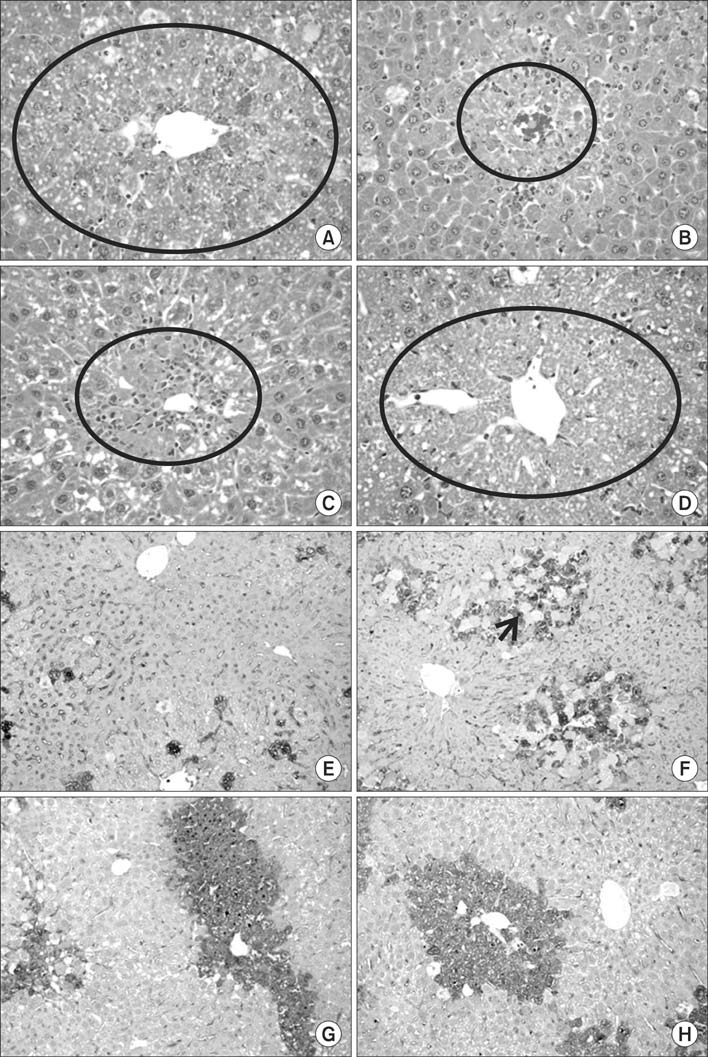
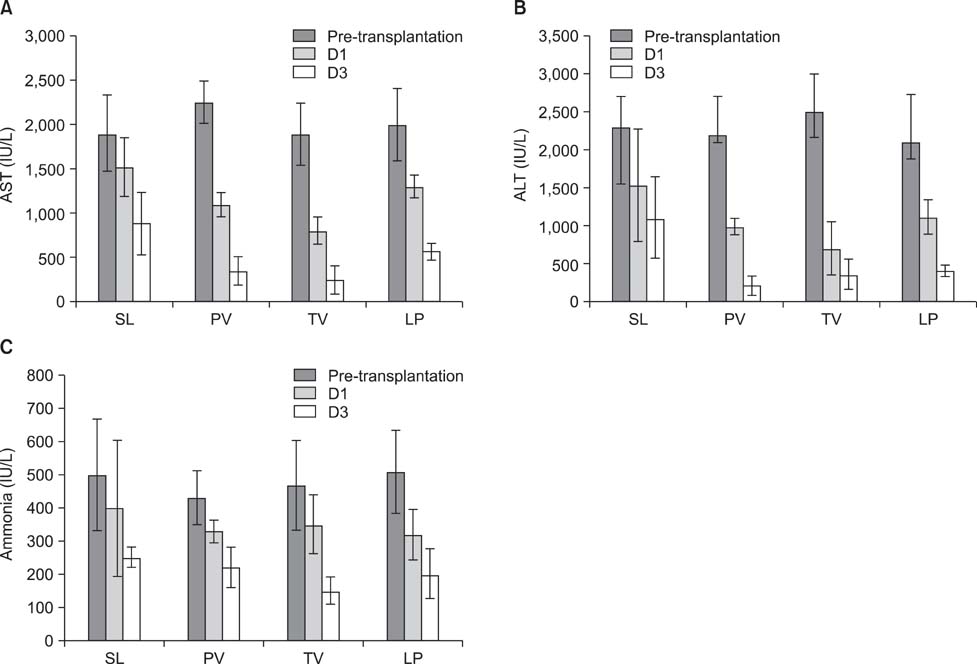
We have shown here that ADSCs have the characteristics of mesenchymal stem cells, and belong to endodermal and/or early hepatic differentiation stage. After transplantation into the mice with acute liver failure, markers of liver injury, such as alanineaminotransferase, aspartateaminotransferase, as well as ammonia, decreased. Of these transplantation routes, transplantation via tail vein rendered the most prominent reduction in the biochemical parameters.
CONCLUSION
Undifferentiated ADSCs have the ability to improve hepatic function in mice with acute liver injury. Moreover, our transplantation route study supports the theory that ADSCs in systemic circulation can exert endocrine or paracrine effects to ameliorate the injured liver.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Human Adipose-Tissue Derived Stem Cell Infusion on the Immunological Consequences in Skin Allograft Mice
Sang Chul Lee, Haejoung Sul, Sang-Mook Lee, Say-June Kim
J Korean Soc Transplant. 2013;27(4):174-184. doi: 10.4285/jkstn.2013.27.4.174.
Reference
-
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998. 282:1145–1147.2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.3. McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006. 24:1246–1253.4. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.5. Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999. 181:67–73.6. De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007. 25:100–106.7. Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004. 22:625–634.8. Shih DT, Lee DC, Chen SC, Tsai RY, Huang CT, Tsai CC, et al. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005. 23:1012–1020.9. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.10. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.11. Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005. 328:258–264.12. Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009. 24:70–77.13. Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008. 26:2705–2712.14. Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007. 46:219–228.15. Liang L, Ma T, Chen W, Hu J, Bai X, Li J, et al. Therapeutic potential and related signal pathway of adipose-derived stem cell transplantation for rat liver injury. Hepatol Res. 2009. 39:822–832.16. Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005. 106:756–763.17. Sgodda M, Aurich H, Kleist S, Aurich I, König S, Dollinger MM, et al. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007. 313:2875–2886.18. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004. 109:1292–1298.19. Lee SW, Min SO, Kim SY, Choi SB, Kim HO, Kim KS. Mesenchymal stem cells: the promotion of endodermal-induction using activin A. Korean J Hepatobiliary Pancreat Surg. 2009. 13:205–214.20. Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999. 284:1168–1170.21. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004. 36:568–584.22. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005. 54:132–141.23. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006. 24:1294–1301.24. Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007. 56:405–415.25. De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004. 104:3472–3482.26. Gilchrist ES, Plevris JN. Bone marrow-derived stem cells in liver repair: 10 years down the line. Liver Transpl. 2010. 16:118–129.27. Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001. 97:3354–3360.28. Mavier P, Martin N, Couchie D, Préaux AM, Laperche Y, Zafrani ES. Expression of stromal cell-derived factor-1 and of its receptor CXCR4 in liver regeneration from oval cells in rat. Am J Pathol. 2004. 165:1969–1977.29. Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999. 283:845–848.30. Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997. 185:111–120.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose-derived stem cells: characterization and clinical application
- Stem Cell Properties of Therapeutic Potential
- Adipose Tissue - Adequate, Accessible Regenerative Material
- Hepatic Stem Cell Transplantation in Liver Failure
- Advanced Research on Stem Cell Therapy for Hepatic Diseases: Potential Implications of a Placenta-derived Mesenchymal Stem Cell-based Strategy