Korean J Gastroenterol.
2011 Sep;58(3):125-132. 10.4166/kjg.2011.58.3.125.
Stem Cell Properties of Therapeutic Potential
- Affiliations
-
- 1Department of Internal Medicine, Digestive Disease Research Institute, Wonkwang University College of Medicine, Iksan, Korea. medsgs@wonkwang.ac.kr
- KMID: 1018399
- DOI: http://doi.org/10.4166/kjg.2011.58.3.125
Abstract
- Stem cell research is a innovative technology that focuses on using undifferentiated cells able to self-renew through the asymmetrical or symmetrical divisions. Three types of stem cells have been studied in laboratory including embryonic stem cell, adult stem cells and induced pluripotent stem cells. Embryonic stem cells are pluripotent stem cells derived from the inner cell mass and it can give rise to any fetal or adult cell type. Adult stem cells are multipotent, have the ability to differentiate into a limited number of specialized cell types, and have been obtained from the bone marrow, umbilical cord blood, placenta and adipose tissue. Stem cell therapy is the most promising therapy for several degenerative and devastating diseases including digestive tract disease such as liver failure, inflammatory bowel disease, Celiac sprue, and pancreatitis. Further understanding of biological properties of stem cells will lead to safe and successful stem cell therapies.
MeSH Terms
Figure
Reference
-
References
1. Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997; 88:287–298.
Article2. Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008; 111:492–503.
Article3. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147.
Article4. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981; 78:7634–7638.
Article5. Chung HM. Clinical application of human embryonic stem cells. J Korean Med Assoc. 2011; 54:454–461.
Article6. Hattori N, Nishimo K, Ko YG, et al. Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. J Biol Chem. 2004; 279:17063–17069.
Article7. Jackson M, Krassowska A, Gilbert N, et al. Severe global DNA hypomethylation blocks differentiation and induces histone hyper-acetylation in embryonic stem cells. Mol Cell Biol. 2004; 24:8862–8871.
Article8. Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell specific MicroRNAs. Dev Cell. 2003; 5:351–358.9. Suh MR, Lee Y, Kim JY, et al. Human embryonic stem cells express a unique set of microRNAs. Dev Biol. 2004; 270:488–498.
Article10. Carpenter MK, Rosler ES, Fisk GJ, et al. Properties of four human embryonic stem cell lines maintained in a feeder-free culture system. Dev Dyn. 2004; 229:243–258.
Article11. Cheong C, Hong KU, Lee HW. Mouse models for telomere and telomerase biology. Exp Mol Med. 2003; 35:141–153.
Article12. Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified poly-peptides. Nature. 1988; 336:688–690.
Article13. Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988; 336:684–687.
Article14. Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004; 10:55–63.
Article15. Bhattacharya B, Miura T, Brandenberger R, et al. Gene expression in human embryonic stem cell lines: unique molecular signature. Blood. 2004; 103:2956–2964.
Article16. Chen Y, Li X, Eswarakumar VP, Seger R, Lonai P. Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene. 2000; 19:3750–3756.
Article17. Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994; 8:3045–3057.
Article18. Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000; 227:271–278.
Article19. Song MR, Ghosh A. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat Neurosci. 2004; 7:229–235.
Article20. Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005; 24:5713–5721.
Article21. Pesce M, Schöler HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001; 19:271–278.22. Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000; 24:372–376.
Article23. Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003; 113:643–655.
Article24. Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003; 113:631–642.
Article25. Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004; 23:7150–7160.
Article26. Zhang X, Yalcin S, Lee DF, et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol. 2011. [Epub ahead of print].
Article27. Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000; 18:399–404.
Article28. Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961; 14:213–222.
Article29. Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001; 2:75–82.
Article30. Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000; 6:1229–1234.
Article31. Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angio-blasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001; 7:430–436.
Article32. Gallacher L, Murdoch B, Wu DM, Karanu FN, Keeney M, Bhatia M. Isolation and characterization of human CD34(−)Lin(−) and CD34(+)Lin(−) hematopoietic stem cells using cell surface markers AC133 and CD7. Blood. 2000; 95:2813–2820.
Article33. Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001; 105:829–841.34. Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997; 276:71–74.
Article35. Kim YI, Oh IH. Cell biological characteristics of adult stem cells. J Korean Med Assoc. 2005; 48:993–1002.
Article36. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676.
Article37. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131:861–872.
Article38. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997; 385:810–813.
Article39. Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogram-ming of somatic cells after fusion with human embryonic stem cells. Science. 2005; 309:1369–1373.
Article40. Yoon BS, You S. Trends and clinical application of induced pluripotent stem cells. J Korean Med Assoc. 2011; 54:502–510.
Article41. Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999; 4:D286–D298.
Article42. Piscaglia AC, Novi M, Campanale M, Gasbarrini A. Stem cellbased therapy in gastroenterology and hepatology. Minim Invasive Ther Allied Technol. 2008; 17:100–118.
Article43. Wong WM, Wright NA. Cell proliferation in gastrointestinal mucosa. J Clin Pathol. 1999; 52:321–333.
Article44. Brittan M, Wright NA. Gastrointestinal stem cells. J Pathol. 2002; 197:492–509.
Article45. Brittan M, Wright NA. The gastrointestinal stem cell. Cell Prolif. 2004; 37:35–53.
Article46. Körbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002; 346:738–746.
Article47. Krause DS, Theise ND, Collector MI, et al. Multiorgan, multilineage engraftment by a single bone marrow-derived stem cell. Cell. 2001; 105:369–377.
Article48. Khalil PN, Weiler V, Nelson PJ, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007; 132:944–954.
Article49. Sanders KM. Interstitial cells of Cajal at the clinical and scientific interface. J Physiol. 2006; 576:683–687.
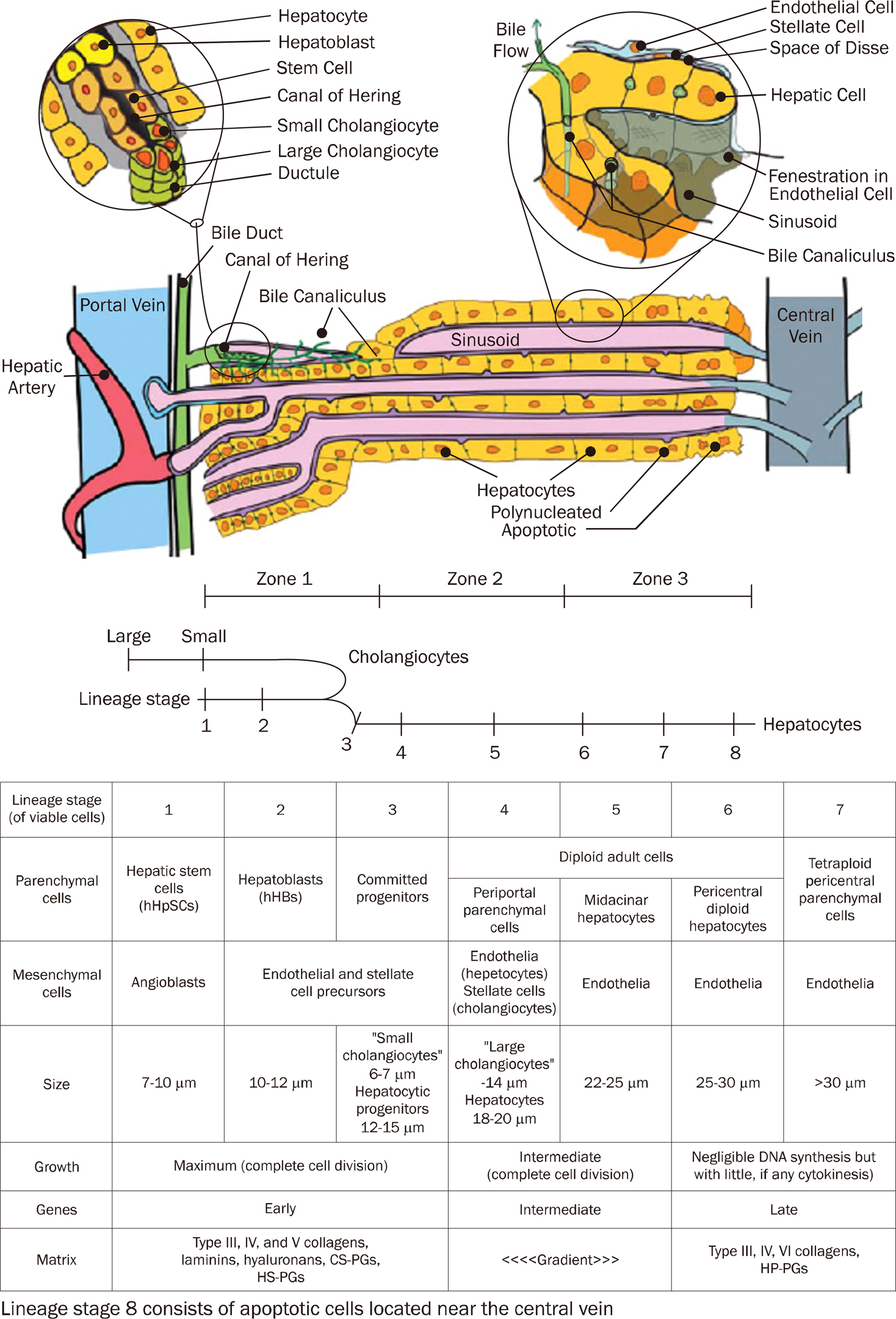
Article50. Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011; 53:1035–1045.
Article51. Zajicek G, Oren R, Weinreb M Jr. The streaming liver. Liver. 1985; 5:293–300.
Article52. Zajicek G, Schwartz-Arad D, Bartfeld E. The streaming liver. V: Time and age-dependent changes of hepatocyte DNA content, following partial hepatectomy. Liver. 1989; 9:164–171.
Article53. Bralet MP, Branchereau S, Brechot C, Ferry N. Cell lineage study in the liver using retroviral mediated gene transfer. Evidence against the streaming of hepatocytes in normal liver. Am J Pathol. 1994; 144:896–905.54. Stowell RE, Lee CS. Histochemical studies of mouse liver after single feeding of carbon tetrachloride. AMA Arch Pathol. 1950; 50:519–537.55. Bae SH. Clinical application of stem cells in liver diseases. Korean J Hepatol. 2008; 14:309–317.
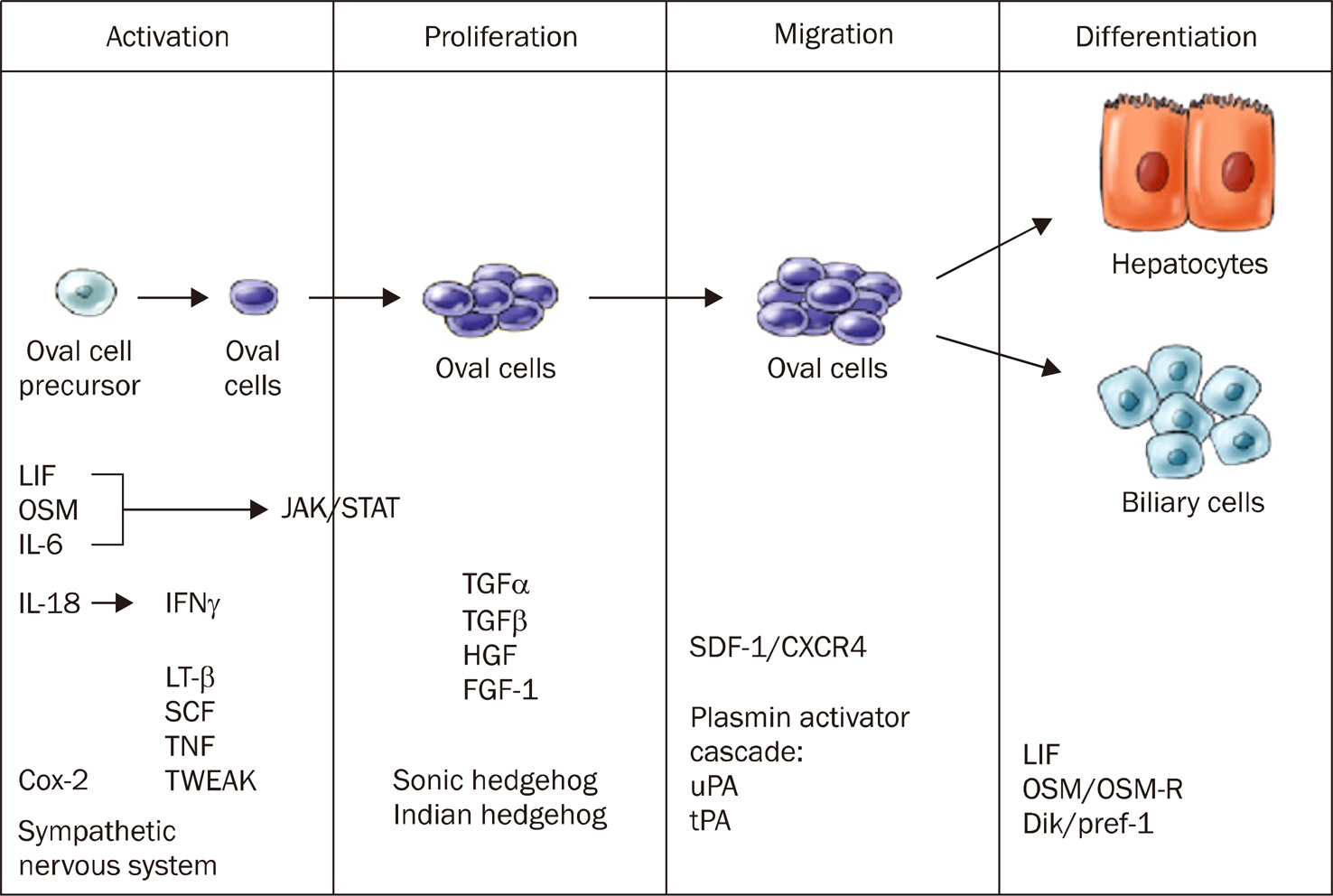
Article56. Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009; 137:466–481.
Article57. Theise ND, Badve S, Saxena R, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000; 31:235–240.
Article58. Alison MR, Poulsom R, Jeffery R, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000; 406:257.
Article59. Ng IO, Chan KL, Shek WH, et al. High frequency of chimerism in transplanted livers. Hepatology. 2003; 38:989–998.
Article60. Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002; 297:2256–2259.
Article61. Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002; 416:542–545.
Article62. Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000; 97:7999–8004.
Article63. Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulindependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000; 6:278–282.
Article64. Seaberg RM, Smukler SR, Kieffer TJ, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004; 22:1115–1124.
Article65. Liu T, Wang C, Wan C, Xiong J, Xu Y, Zhou F. PDX-1 expression in pancreatic ductal cells after partial pancreatectomy in adult rats. J Huazhong Univ Sci Technolog Med Sci. 2004; 24:464–466.66. Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulindependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000; 6:278–282.
Article67. Zulewski H, Abraham EJ, Gerlach MJ, et al. Multipotential nes-tin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001; 50:521–533.
Article68. Lechner A, Leech CA, Abraham EJ, Nolan AL, Habener JF. Nestin-positive progenitor cells derived from adult human pancreatic islets of Langerhans contain side population (SP) cells defined by expression of the ABCG2 (BCRP1) ATP-binding cas-sette transporter. Biochem Biophys Res Commun. 2002; 293:670–674.
Article69. Wang R, Yashpal N, Bacchus F, Li J. Hepatocyte growth factor regulates proliferation and differentiation of epithelial monolayers derived from islets of postnatal rat pancreas. J Endocrinol. 2004; 183:163–171.
Article70. Yashpal NK, Li J, Wang R. Characterization of c-Kit and nestin expression during islet cell development in the prenatal and postnatal rat pancreas. Dev Dyn. 2004; 229:813–825.
Article71. Wang R, Li J, Yashpal N. Phenotypic analysis of c-Kit expression in epithelial monolayers derived from postnatal rat pancreatic islets. J Endocrinol. 2004; 182:113–122.
Article72. Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005; 54:2568–2575.73. Kojima H, Fujimiya M, Matsumura K, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003; 9:596–603.
Article74. Timper K, Seboek D, Eberhardt M, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006; 341:1135–1140.
Article75. Fujita Y, Cheung AT, Kieffer TJ. Harnessing the gut to treat diabetes. Pediatr Diabetes. 2004; 5(Suppl 2):57–69.
Article76. Jung KH, Song SU, Yi T, et al. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011; 140:998–1008.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Applications of Stem Cells in Regenerative Medicine
- Targeting Cancer Stem Cell Markers or Pathways: A Potential Therapeutic Strategy for Oral Cancer Treatment
- Adult Stem Cell Therapy in Chronic Liver Diseases
- Delivering Factors for Reprogramming a Somatic Cell to Pluripotency
- The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation