Int J Stem Cells.
2019 Mar;12(1):8-20. 10.15283/ijsc18109.
Role of HIF1α Regulatory Factors in Stem Cells
- Affiliations
-
- 1Department of Veterinary Physiology, College of Veterinary Medicine, Research Institute for Veterinary Science, and BK21 PLUS Program for Creative Veterinary Science Research, Seoul National University, Seoul, Korea. hjhan@snu.ac.kr
- KMID: 2447220
- DOI: http://doi.org/10.15283/ijsc18109
Abstract
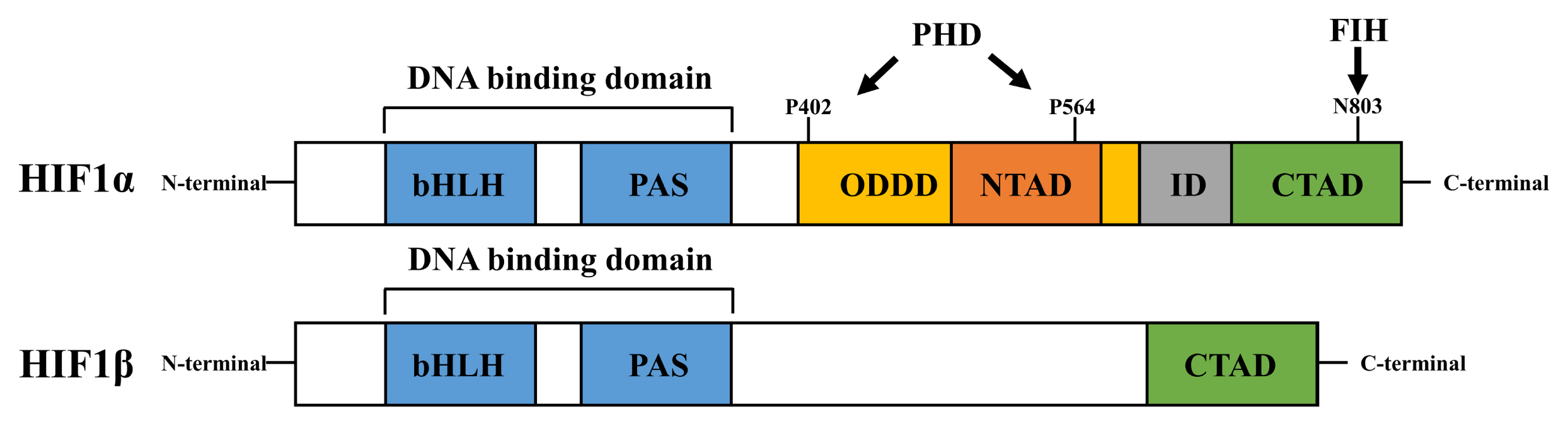
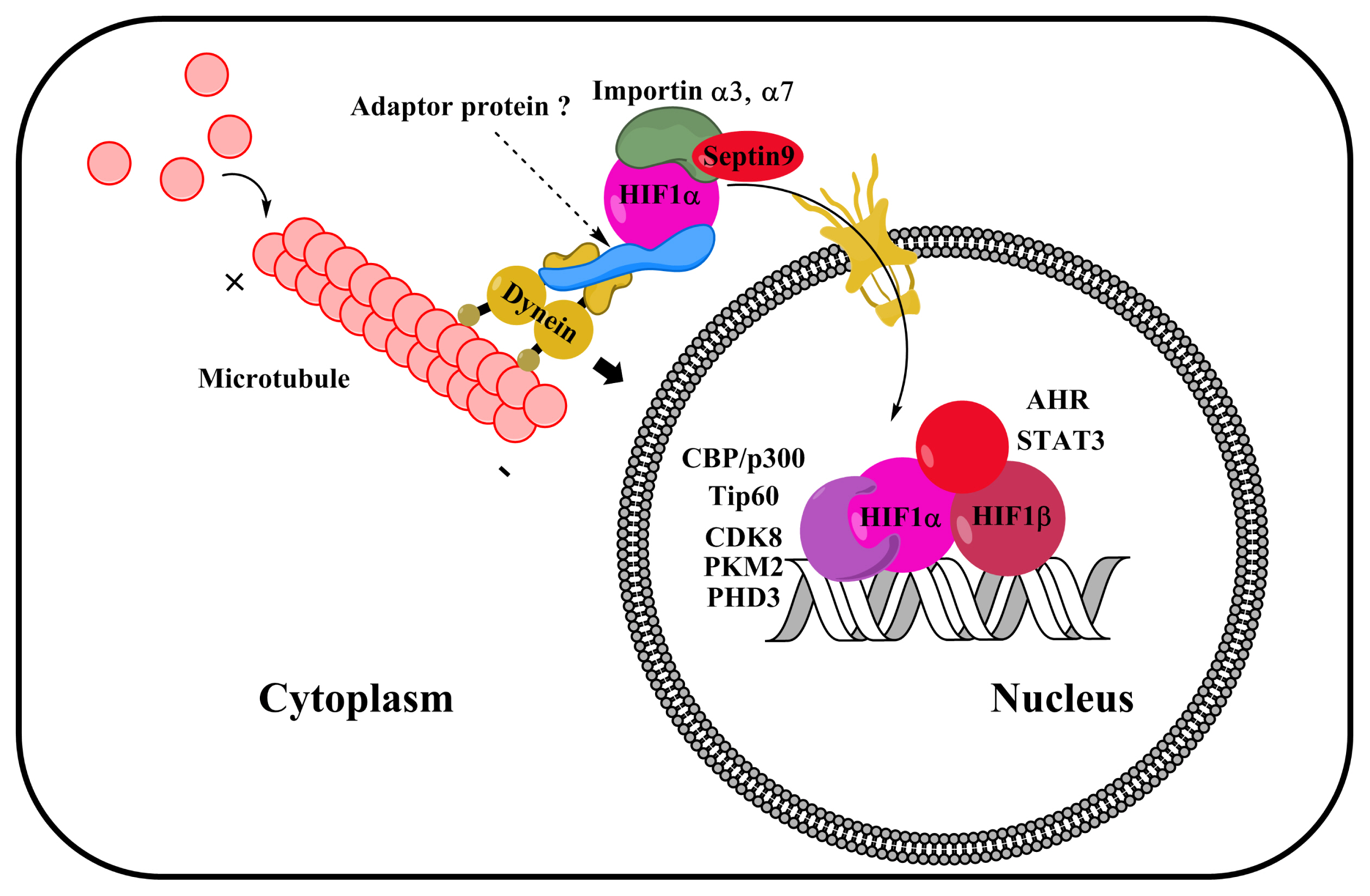
- Hypoxia-inducible factor 1 (HIF1) is a master transcription factor that induces the transcription of genes involved in the metabolism and behavior of stem cells. HIF1-mediated adaptation to hypoxia is required to maintain the pluripotency and survival of stem cells under hypoxic conditions. HIF1 activity is well known to be tightly controlled by the alpha subunit of HIF1 (HIF1α). Understanding the regulatory mechanisms that control HIF1 activity in stem cells will provide novel insights into stem cell biology under hypoxia. Recent research has unraveled the mechanistic details of HIF1α regulating processes, suggesting new strategies for regulating stem cells. This review summarizes recent experimental studies on the role of several regulatory factors (including calcium, 2-oxoglutarate-dependent dioxygenase, microtubule network, importin, and coactivators) in regulating HIF1α activity in stem cells.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Zhang CC, Sadek HA. Hypoxia and metabolic properties of hematopoietic stem cells. Antioxid Redox Signal. 2014; 20:1891–1901. DOI: 10.1089/ars.2012.5019. PMID: 23621582. PMCID: 3967354.
Article2. Chen J, Kang JG, Keyvanfar K, Young NS, Hwang PM. Long-term adaptation to hypoxia preserves hematopoietic stem cell function. Exp Hematol. 2016; 44:866–873.e4. DOI: 10.1016/j.exphem.2016.04.010. PMID: 27118043.
Article3. Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab. 2018; 27:281–298. DOI: 10.1016/j.cmet.2017.10.005. PMID: 29129785.
Article4. Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR. Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell. 2016; 19:476–490. DOI: 10.1016/j.stem.2016.08.008. PMID: 27618217. PMCID: 5055460.
Article5. Seo BN, Ryu JM, Yun SP, Jeon JH, Park SS, Oh KB, Park JK, Han HJ. Delphinidin prevents hypoxia-induced mouse embryonic stem cell apoptosis through reduction of intracellular reactive oxygen species-mediated activation of JNK and NF-κB, and Akt inhibition. Apoptosis. 2013; 18:811–824. DOI: 10.1007/s10495-013-0838-2. PMID: 23584725.
Article6. Son TW, Yun SP, Yong MS, Seo BN, Ryu JM, Youn HY, Oh YM, Han HJ. Netrin-1 protects hypoxia-induced mitochondrial apoptosis through HSP27 expression via DCC-and integrin α6β4-dependent Akt, GSK-3β, and HSF-1 in mesenchymal stem cells. Cell Death Dis. 2013; 4:e563. DOI: 10.1038/cddis.2013.94. PMID: 23538444. PMCID: 3615739.7. Bader AM, Klose K, Bieback K, Korinth D, Schneider M, Seifert M, Choi YH, Kurtz A, Falk V, Stamm C. Hypoxic preconditioning increases survival and pro-angiogenic capacity of human cord blood mesenchymal stromal cells in vitro. PLoS One. 2015; 10:e0138477. DOI: 10.1371/journal.pone.0138477. PMID: 26380983. PMCID: 4575058.
Article8. Liu YY, Chiang CH, Hung SC, Chian CF, Tsai CL, Chen WC, Zhang H. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS One. 2017; 12:e0187637. DOI: 10.1371/journal.pone.0187637. PMID: 29117205. PMCID: 5678873.
Article9. Lee HJ, Ryu JM, Jung YH, Oh SY, Lee SJ, Han HJ. Novel pathway for hypoxia-induced proliferation and migration in human mesenchymal stem cells: involvement of HIF-1 α, FASN, and mTORC1. Stem Cells. 2015; 33:2182–2195. DOI: 10.1002/stem.2020. PMID: 25825864.
Article10. Lee HJ, Jung YH, Choi GE, Ko SH, Lee SJ, Lee SH, Han HJ. BNIP3 induction by hypoxia stimulates FASN-dependent free fatty acid production enhancing therapeutic potential of umbilical cord blood-derived human mesenchymal stem cells. Redox Biol. 2017; 13:426–443. DOI: 10.1016/j.redox.2017.07.004. PMID: 28704726. PMCID: 5508529.
Article11. Lee HJ, Ryu JM, Jung YH, Lee KH, Kim DI, Han HJ. Glycerol-3-phosphate acyltransferase-1 upregulation by O-GlcNAcylation of Sp1 protects against hypoxia-induced mouse embryonic stem cell apoptosis via mTOR activation. Cell Death Dis. 2016; 7:e2158. DOI: 10.1038/cddis.2015.410. PMID: 27010859. PMCID: 4823928.
Article12. Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991; 88:5680–5684. DOI: 10.1073/pnas.88.13.5680. PMID: 2062846. PMCID: 51941.
Article13. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995; 92:5510–5514. DOI: 10.1073/pnas.92.12.5510. PMID: 7539918. PMCID: 41725.
Article14. Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992; 256:1193–1195. DOI: 10.1126/science.256.5060.1193. PMID: 1317062.
Article15. Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1α. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997; 272:19253–19260. DOI: 10.1074/jbc.272.31.19253. PMID: 9235919.16. Azimi I. The interplay between HIF-1 and calcium signalling in cancer. Int J Biochem Cell Biol. 2018; 97:73–77. DOI: 10.1016/j.biocel.2018.02.001. PMID: 29407528.
Article17. Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1α transcriptional activity. Genes Dev. 2001; 15:2675–2686. DOI: 10.1101/gad.924501. PMID: 11641274. PMCID: 312814.
Article18. Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002; 16:1466–1471. DOI: 10.1101/gad.991402. PMID: 12080085. PMCID: 186346.
Article19. Semenza GL. HIF-1 mediates metabolic responses to intra-tumoral hypoxia and oncogenic mutations. J Clin Invest. 2013; 123:3664–3671. DOI: 10.1172/JCI67230. PMID: 23999440. PMCID: 3754249.
Article20. Man J, Yu X, Huang H, Zhou W, Xiang C, Huang H, Miele L, Liu Z, Bebek G, Bao S, Yu JS. Hypoxic induction of vasorin regulates Notch1 turnover to maintain glioma stem-like cells. Cell Stem Cell. 2018; 22:104–118.e6. DOI: 10.1016/j.stem.2017.10.005. PMID: 29198941. PMCID: 5756127.
Article21. Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001; 13:167–171. DOI: 10.1016/S0955-0674(00)00194-0. PMID: 11248550.
Article22. Lee JH, Han YS, Yoon YM, Yun CW, Yun SP, Kim SM, Kwon HY, Jeong D, Baek MJ, Lee HJ, Lee SJ, Han HJ, Lee SH. Role of HSPA1L as a cellular prion protein stabilizer in tumor progression via HIF-1α/GP78 axis. Oncogene. 2017; 36:6555–6567. DOI: 10.1038/onc.2017.263. PMID: 28759037.
Article23. Lee HJ, Ryu JM, Jung YH, Lee SJ, Kim JY, Lee SH, Hwang IK, Seong JK, Han HJ. High glucose upregulates BACE1-mediated Aβ production through ROS-dependent HIF-1α and LXRα/ABCA1-regulated lipid raft reorganization in SK-N-MC cells. Sci Rep. 2016; 6:36746. DOI: 10.1038/srep36746. PMID: 27829662. PMCID: 5103190.
Article24. Menendez-Montes I, Escobar B, Palacios B, Gómez MJ, Izquierdo-Garcia JL, Flores L, Jiménez-Borreguero LJ, Aragones J, Ruiz-Cabello J, Torres M, Martin-Puig S. Myocardial VHL-HIF signaling controls an embryonic metabolic switch essential for cardiac maturation. Dev Cell. 2016; 39:724–739. DOI: 10.1016/j.devcel.2016.11.012. PMID: 27997827.
Article25. Lee JH, Yoon YM, Lee SH. Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF-1α-GRP78-Akt axis. Int J Mol Sci. 2017; 18:DOI: 10.3390/ijms18061320. PMID: 28635661. PMCID: 5486141.
Article26. Yun SP, Lee MY, Ryu JM, Song CH, Han HJ. Role of HIF-1α and VEGF in human mesenchymal stem cell proliferation by 17β-estradiol: involvement of PKC, PI3K/Akt, and MAPKs. Am J Physiol Cell Physiol. 2009; 296:C317–326. DOI: 10.1152/ajpcell.00415.2008. PMID: 18987249.
Article27. Forristal CE, Nowlan B, Jacobsen RN, Barbier V, Walkinshaw G, Walkley CR, Winkler IG, Levesque JP. HIF-1α is required for hematopoietic stem cell mobilization and 4-prolyl hydroxylase inhibitors enhance mobilization by stabilizing HIF-1α. Leukemia. 2015; 29:1366–1378. DOI: 10.1038/leu.2015.8. PMID: 25578474. PMCID: 4498452.
Article28. Lee SH, Suh HN, Lee YJ, Seo BN, Ha JW, Han HJ. Midkine prevented hypoxic injury of mouse embryonic stem cells through activation of Akt and HIF-1α via low-density lipoprotein receptor-related protein-1. J Cell Physiol. 2012; 227:1731–1739. DOI: 10.1002/jcp.22897. PMID: 21688265.
Article29. Lee SH, Lee YJ, Han HJ. Effect of arachidonic acid on hypoxia-induced IL-6 production in mouse ES cells: involvement of MAPKs, NF-κB, and HIF-1α. J Cell Physiol. 2010; 222:574–585. PMID: 19950212. PMCID: 10.1002/jcp.21973.
Article30. Lee SH, Kim MH, Han HJ. Arachidonic acid potentiates hypoxia-induced VEGF expression in mouse embryonic stem cells: involvement of Notch, Wnt, and HIF-1α. Am J Physiol Cell Physiol. 2009; 297:C207–216. DOI: 10.1152/ajpcell.00579.2008. PMID: 19339510.
Article31. Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998; 8:588–594. DOI: 10.1016/S0959-437X(98)80016-6. PMID: 9794818.32. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003; 3:721–732. DOI: 10.1038/nrc1187. PMID: 13130303.
Article33. Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015; 5:378–389. DOI: 10.1016/j.apsb.2015.05.007. PMID: 26579469. PMCID: 4629436.
Article34. Dengler VL, Galbraith M, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014; 49:1–15. DOI: 10.3109/10409238.2013.838205. PMID: 24099156. PMCID: 4342852.
Article35. Ernens I, Goodfellow SJ, Innes F, Kenneth NS, Derblay LE, White RJ, Scott PH. Hypoxic stress suppresses RNA polymerase III recruitment and tRNA gene transcription in cardiomyocytes. Nucleic Acids Res. 2006; 34:286–294. DOI: 10.1093/nar/gkj402. PMID: 16407335. PMCID: 1326236.
Article36. Minet E, Ernest I, Michel G, Roland I, Remacle J, Raes M, Michiels C. HIF1A gene transcription is dependent on a core promoter sequence encompassing activating and inhibiting sequences located upstream from the transcription initiation site and cis elements located within the 5′UTR. Biochem Biophys Res Commun. 1999; 261:534–540. DOI: 10.1006/bbrc.1999.0995. PMID: 10425220.
Article37. Belaiba RS, Bonello S, Zähringer C, Schmidt S, Hess J, Kietzmann T, Görlach A. Hypoxia up-regulates hypoxia-inducible factor-1α transcription by involving phosphatidylinositol 3-kinase and nuclear factor κB in pulmonary artery smooth muscle cells. Mol Biol Cell. 2007; 18:4691–4697. DOI: 10.1091/mbc.e07-04-0391. PMID: 17898080. PMCID: 2096613.
Article38. Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I, Szabadkai G, Rizzuto R, Mammucari C. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Mol Med. 2016; 8:569–585. DOI: 10.15252/emmm.201606255. PMID: 27138568. PMCID: 4864890.
Article39. Chamboredon S, Ciais D, Desroches-Castan A, Savi P, Bono F, Feige JJ, Cherradi N. Hypoxia-inducible factor-1 α mRNA: a new target for destabilization by tristetraprolin in endothelial cells. Mol Biol Cell. 2011; 22:3366–3378. DOI: 10.1091/mbc.e10-07-0617. PMID: 21775632. PMCID: 3172262.
Article40. Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010; 139:85–97. DOI: 10.1530/REP-09-0300. PMID: 19755485. PMCID: 2791494.
Article41. Lv B, Li F, Fang J, Xu L, Sun C, Han J, Hua T, Zhang Z, Feng Z, Jiang X. Hypoxia inducible factor 1α promotes survival of mesenchymal stem cells under hypoxia. Am J Transl Res. 2017; 9:1521–1529. PMID: 28386377. PMCID: 5376042.42. Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001; 15:807–826. DOI: 10.1101/gad.887201. PMID: 11297505.
Article43. Harada H, Itasaka S, Kizaka-Kondoh S, Shibuya K, Morinibu A, Shinomiya K, Hiraoka M. The Akt/mTOR pathway assures the synthesis of HIF-1α protein in a glucose-and reoxygenation-dependent manner in irradiated tumors. J Biol Chem. 2009; 284:5332–5342. DOI: 10.1074/jbc.M806653200. PMID: 19098000.
Article44. Dodd KM, Yang J, Shen MH, Sampson JR, Tee AR. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2015; 34:2239–2250. DOI: 10.1038/onc.2014.164. PMID: 24931163. PMCID: 4172452.
Article45. Sakamoto T, Weng JS, Hara T, Yoshino S, Kozuka-Hata H, Oyama M, Seiki M. Hypoxia-inducible factor 1 regulation through cross talk between mTOR and MT1-MMP. Mol Cell Biol. 2014; 34:30–42. DOI: 10.1128/MCB.01169-13. PMID: 24164895. PMCID: 3911284.
Article46. Land SC, Tee AR. Hypoxia-inducible factor 1α is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007; 282:20534–20543. DOI: 10.1074/jbc.M611782200. PMID: 17502379.
Article47. Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG Jr. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004; 18:2893–2904. DOI: 10.1101/gad.1256804. PMID: 15545625. PMCID: 534650.
Article48. Vadysirisack DD, Ellisen LW. mTOR activity under hypoxia. Methods Mol Biol. 2012; 821:45–58. DOI: 10.1007/978-1-61779-430-8_4. PMID: 22125059. PMCID: 3960283.
Article49. Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 2002; 16:771–780. DOI: 10.1096/fj.01-0658com. PMID: 12039858.
Article50. Lv B, Hua T, Li F, Han J, Fang J, Xu L, Sun C, Zhang Z, Feng Z, Jiang X. Hypoxia-inducible factor 1 α protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am J Transl Res. 2017; 9:2492–2499. PMID: 28559999. PMCID: 5446531.51. Koyasu S, Kobayashi M, Goto Y, Hiraoka M, Harada H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018; 109:560–571. DOI: 10.1111/cas.13483. PMID: 29285833. PMCID: 5834787.
Article52. Moreno M, Fernández V, Monllau JM, Borrell V, Lerin C, de la Iglesia N. Transcriptional profiling of hypoxic neural stem cells identifies calcineurin-NFATc4 signaling as a major regulator of neural stem cell biology. Stem Cell Reports. 2015; 5:157–165. DOI: 10.1016/j.stemcr.2015.06.008. PMID: 26235896. PMCID: 4618660.
Article53. Seta KA, Yuan Y, Spicer Z, Lu G, Bedard J, Ferguson TK, Pathrose P, Cole-Strauss A, Kaufhold A, Millhorn DE. The role of calcium in hypoxia-induced signal transduction and gene expression. Cell Calcium. 2004; 36:331–340. DOI: 10.1016/j.ceca.2004.02.006. PMID: 15261489.
Article54. Wang Q, Gao S, Luo Y, Kang QY. Compound anisodine affects the proliferation and calcium overload of hypoxia-induced rat retinal progenitor cells and brain neural stem cells via the p-ERK1/2/HIF-1α/VEGF pathway. Exp Ther Med. 2017; 14:600–608. DOI: 10.3892/etm.2017.4528. PMID: 28672973. PMCID: 5488403.
Article55. Mottet D, Michel G, Renard P, Ninane N, Raes M, Michiels C. Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J Cell Physiol. 2003; 194:30–44. DOI: 10.1002/jcp.10176. PMID: 12447987.
Article56. Corallino S, Malinverno C, Neumann B, Tischer C, Palamidessi A, Frittoli E, Panagiotakopoulou M, Disanza A, Malet-Engra G, Nastaly P, Galli C, Luise C, Bertalot G, Pece S, Di Fiore PP, Gauthier N, Ferrari A, Maiuri P, Scita G. A RAB35-p85/PI3K axis controls oscillatory apical protrusions required for efficient chemotactic migration. Nat Commun. 2018; 9(1):1475. DOI: 10.1038/s41467-018-03571-8. PMID: 29662076. PMCID: 5902610.
Article57. Valsecchi V, Pignataro G, Del Prete A, Sirabella R, Matrone C, Boscia F, Scorziello A, Sisalli MJ, Esposito E, Zambrano N, Di Renzo G, Annunziato L. NCX1 is a novel target gene for hypoxia-inducible factor-1 in ischemic brain preconditioning. Stroke. 2011; 42:754–763. DOI: 10.1161/STROKEAHA.110.597583. PMID: 21293012.
Article58. Li Y, Guo B, Xie Q, Ye D, Zhang D, Zhu Y, Chen H, Zhu B. STIM1 mediates hypoxia-driven hepatocarcinogenesis via interaction with HIF-1. Cell Rep. 2015; 12:388–395. DOI: 10.1016/j.celrep.2015.06.033. PMID: 26166565.
Article59. Wang J, Xu C, Zheng Q, Yang K, Lai N, Wang T, Tang H, Lu W. Orai1, 2, 3 and STIM1 promote store-operated calcium entry in pulmonary arterial smooth muscle cells. Cell Death Discov. 2017; 3:17074. DOI: 10.1038/cddiscovery.2017.74. PMID: 29188077. PMCID: 5702854.
Article60. Xiang L, Chen XJ, Wu KC, Zhang CJ, Zhou GH, Lv JN, Sun LF, Cheng FF, Cai XB, Jin ZB. miR-183/96 plays a pivotal regulatory role in mouse photoreceptor maturation and maintenance. Proc Natl Acad Sci U S A. 2017; 114:6376–6381. DOI: 10.1073/pnas.1618757114. PMID: 28559309. PMCID: 5474811.
Article61. Jenkins J, Papkovsky DB, Dmitriev RI. The Ca2+/Mn2+-transporting SPCA2 pump is regulated by oxygen and cell density in colon cancer cells. Biochem J. 2016; 473:2507–2518. DOI: 10.1042/BCJ20160477. PMID: 27316461.
Article62. Ronkainen VP, Skoumal R, Tavi P. Hypoxia and HIF-1 suppress SERCA2a expression in embryonic cardiac myocytes through two interdependent hypoxia response elements. J Mol Cell Cardiol. 2011; 50:1008–1016. DOI: 10.1016/j.yjmcc.2011.02.017. PMID: 21382378.
Article63. Chen SJ, Hoffman NE, Shanmughapriya S, Bao L, Keefer K, Conrad K, Merali S, Takahashi Y, Abraham T, Hirschler-Laszkiewicz I, Wang J, Zhang XQ, Song J, Barrero C, Shi Y, Kawasawa YI, Bayerl M, Sun T, Barbour M, Wang HG, Madesh M, Cheung JY, Miller BA. A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2α. J Biol Chem. 2014; 289:36284–36302. DOI: 10.1074/jbc.M114.620922. PMID: 25391657. PMCID: 4276889.
Article64. Hui AS, Bauer AL, Striet JB, Schnell PO, Czyzyk-Krzeska MF. Calcium signaling stimulates translation of HIF-α during hypoxia. FASEB J. 2006; 20:466–475. DOI: 10.1096/fj.05-5086com. PMID: 16507764.
Article65. Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008; 217:674–85. DOI: 10.1002/jcp.21537. PMID: 18651560. PMCID: 2696817.66. Asghar MY, Magnusson M, Kemppainen K, Sukumaran P, Löf C, Pulli I, Kalhori V, Törnquist K. Transient Receptor Potential Canonical 1 (TRPC1) channels as regulators of sphingolipid and VEGF receptor expression: implications for thyroid cancer cell migration and proliferation. J Biol Chem. 2015; 290:16116–16131. DOI: 10.1074/jbc.M115.643668. PMID: 25971967. PMCID: 4481213.
Article67. Pchelintseva E, Djamgoz MBA. Mesenchymal stem cell differentiation: control by calcium-activated potassium channels. J Cell Physiol. 2018; 233:3755–3768. DOI: 10.1002/jcp.26120. PMID: 28776687.
Article68. Sun S, Liu Y, Lipsky S, Cho M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007; 21:1472–1480. DOI: 10.1096/fj.06-7153com. PMID: 17264165.
Article69. Jiang LH, Mousawi F, Yang X, Roger S. ATP-induced Ca2+-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cell Mol Life Sci. 2017; 74:3697–3710. DOI: 10.1007/s00018-017-2545-6. PMID: 28534085. PMCID: 5597679.
Article70. Berchner-Pfannschmidt U, Petrat F, Doege K, Trinidad B, Freitag P, Metzen E, de Groot H, Fandrey J. Chelation of cellular calcium modulates hypoxia-inducible gene expression through activation of hypoxia-inducible factor-1α. J Biol Chem. 2004; 279:44976–44986. DOI: 10.1074/jbc.M313995200. PMID: 15322093.
Article71. Yu S, Xu Z, Zou C, Wu D, Wang Y, Yao X, Ng CF, Chan FL. Ion channel TRPM8 promotes hypoxic growth of prostate cancer cells via an O2-independent and RACK1-mediated mechanism of HIF-1α stabilization. J Pathol. 2014; 234:514–525. DOI: 10.1002/path.4413. PMID: 25065497.
Article72. Liu YV, Hubbi ME, Pan F, McDonald KR, Mansharamani M, Cole RN, Liu JO, Semenza GL. Calcineurin promotes hypoxia-inducible factor 1α expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J Biol Chem. 2007; 282:37064–37073. DOI: 10.1074/jbc.M705015200. PMID: 17965024. PMCID: 3754800.
Article73. Li S, Wang J, Wei Y, Liu Y, Ding X, Dong B, Xu Y, Wang Y. Crucial role of TRPC6 in maintaining the stability of HIF-1α in glioma cells under hypoxia. J Cell Sci. 2015; 128:3317–3329. DOI: 10.1242/jcs.173161. PMID: 26187851.74. Bargiela D, Burr SP, Chinnery PF. Mitochondria and hypoxia: metabolic crosstalk in cell-fate decisions. Trends Endocrinol Metab. 2018; 29:249–259. DOI: 10.1016/j.tem.2018.02.002. PMID: 29501229.
Article75. Clifton IJ, McDonough MA, Ehrismann D, Kershaw NJ, Granatino N, Schofield CJ. Structural studies on 2-oxoglutarate oxygenases and related double-stranded β-helix fold proteins. J Inorg Biochem. 2006; 100:644–669. DOI: 10.1016/j.jinorgbio.2006.01.024. PMID: 16513174.
Article76. Hewitson KS, Liénard BM, McDonough MA, Clifton IJ, Butler D, Soares AS, Oldham NJ, McNeill LA, Schofield CJ. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J Biol Chem. 2007; 282:3293–3301. DOI: 10.1074/jbc.M608337200. PMID: 17135241.
Article77. Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P, Rötig A, Jeunemaitre X. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet. 2001; 69:1186–1197. DOI: 10.1086/324413. PMID: 11605159. PMCID: 1235531.
Article78. Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM, Neckers L. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005; 8:143–153. DOI: 10.1016/j.ccr.2005.06.017. PMID: 16098467.
Article79. Tretter L, Patocs A, Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta. 2016; 1857:1086–1101. DOI: 10.1016/j.bbabio.2016.03.012. PMID: 26971832.
Article80. Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015; 518:413–416. DOI: 10.1038/nature13981. PMID: 25487152. PMCID: 4336218.
Article81. Hwang IY, Kwak S, Lee S, Kim H, Lee SE, Kim JH, Kim YA, Jeon YK, Chung DH, Jin X, Park S, Jang H, Cho EJ, Youn HD. Psat1-dependent fluctuations in α-ketoglutarate affect the timing of ESC differentiation. Cell Metab. 2016; 24:494–501. DOI: 10.1016/j.cmet.2016.06.014. PMID: 27476977.
Article82. TeSlaa T, Chaikovsky AC, Lipchina I, Escobar SL, Hochedlinger K, Huang J, Graeber TG, Braas D, Teitell MA. α-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016; 24:485–493. DOI: 10.1016/j.cmet.2016.07.002. PMID: 27476976. PMCID: 5023506.
Article83. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998; 95:11715–11720. DOI: 10.1073/pnas.95.20.11715. PMID: 9751731. PMCID: 21706.
Article84. Lee G, Won HS, Lee YM, Choi JW, Oh TI, Jang JH, Choi DK, Lim BO, Kim YJ, Park JW, Puigserver P, Lim JH. Oxidative dimerization of PHD2 is responsible for its inactivation and contributes to metabolic reprogramming via HIF-1α activation. Sci Rep. 2016; 6:18928. DOI: 10.1038/srep18928. PMID: 26740011. PMCID: 4703963.
Article85. Siegert I, Schödel J, Nairz M, Schatz V, Dettmer K, Dick C, Kalucka J, Franke K, Ehrenschwender M, Schley G, Beneke A, Sutter J, Moll M, Hellerbrand C, Wielockx B, Katschinski DM, Lang R, Galy B, Hentze MW, Koivunen P, Oefner PJ, Bogdan C, Weiss G, Willam C, Jantsch J. Ferritin-mediated iron sequestration stabilizes hypoxia-inducible factor-1α upon LPS activation in the presence of ample oxygen. Cell Rep. 2015; 13:2048–2055. DOI: 10.1016/j.celrep.2015.11.005. PMID: 26628374.
Article86. Masson N, Singleton RS, Sekirnik R, Trudgian DC, Ambrose LJ, Miranda MX, Tian YM, Kessler BM, Schofield CJ, Ratcliffe PJ. The FIH hydroxylase is a cellular peroxide sensor that modulates HIF transcriptional activity. EMBO Rep. 2012; 13:251–257. DOI: 10.1038/embor.2012.9. PMID: 22310300. PMCID: 3323130.
Article87. Tarhonskaya H, Hardy AP, Howe EA, Loik ND, Kramer HB, McCullagh JS, Schofield CJ, Flashman E. Kinetic investigations of the role of factor inhibiting hypoxia-inducible factor (FIH) as an oxygen sensor. J Biol Chem. 2015; 290:19726–19742. DOI: 10.1074/jbc.M115.653014. PMID: 26112411. PMCID: 4528135.
Article88. Bracken CP, Fedele AO, Linke S, Balrak W, Lisy K, Whitelaw ML, Peet DJ. Cell-specific regulation of hypoxia-inducible factor (HIF)-1α and HIF-2α stabilization and transactivation in a graded oxygen environment. J Biol Chem. 2006; 281:22575–22585. DOI: 10.1074/jbc.M600288200. PMID: 16760477.
Article89. Koivunen P, Hirsilä M, Günzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004; 279:9899–9904. DOI: 10.1074/jbc.M312254200. PMID: 14701857.
Article90. Sim J, Cowburn AS, Palazon A, Madhu B, Tyrakis PA, Macías D, Bargiela DM, Pietsch S, Gralla M, Evans CE, Kittipassorn T, Chey YCJ, Branco CM, Rundqvist H, Peet DJ, Johnson RS. The factor inhibiting HIF asparaginyl hydroxylase regulates oxidative metabolism and accelerates metabolic adaptation to hypoxia. Cell Metab. 2018; 27:898–913.e7. DOI: 10.1016/j.cmet.2018.02.020. PMID: 29617647. PMCID: 5887987.
Article91. Kuiper C, Dachs GU, Currie MJ, Vissers MC. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free Radic Biol Med. 2014; 69:308–317. DOI: 10.1016/j.freeradbiomed.2014.01.033. PMID: 24495550.
Article92. Miles SL, Fischer AP, Joshi SJ, Niles RM. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer. 2015; 15:867. DOI: 10.1186/s12885-015-1878-5. PMID: 26547841. PMCID: 4636772.
Article93. Peng WX, Pan FY, Liu XJ, Ning S, Xu N, Meng FL, Wang YQ, Li CJ. Hypoxia stabilizes microtubule networks and decreases tumor cell chemosensitivity to anticancer drugs through Egr-1. Anat Rec (Hoboken). 2010; 293:414–420. DOI: 10.1002/ar.21086. PMID: 20169563.
Article94. Yoon SO, Shin S, Mercurio AM. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the α6β4 integrin. Cancer Res. 2005; 65:2761–2769. DOI: 10.1158/0008-5472.CAN-04-4122. PMID: 15805276.
Article95. Fang YD, Xu X, Dang YM, Zhang YM, Zhang JP, Hu JY, Zhang Q, Dai X, Teng M, Zhang DX, Huang YS. MAP4 mechanism that stabilizes mitochondrial permeability transition in hypoxia: microtubule enhancement and DYNLT1 interaction with VDAC1. PLoS One. 2011; 6:e28052. DOI: 10.1371/journal.pone.0028052. PMID: 22164227. PMCID: 3229508.
Article96. Hu JY, Chu ZG, Han J, Dang YM, Yan H, Zhang Q, Liang GP, Huang YS. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell Mol Life Sci. 2010; 67:321–333. DOI: 10.1007/s00018-009-0187-z. PMID: 19915797.
Article97. Guo H, Zheng H, Wu J, Ma HP, Yu J, Yiliyaer M. The key role of microtubules in hypoxia preconditioning-induced nuclear translocation of HIF-1α in rat cardiomyocytes. PeerJ. 2017; 5:e3662. DOI: 10.7717/peerj.3662. PMID: 28828258. PMCID: 5560226.
Article98. McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008; 1785:96–132. DOI: 10.1016/j.bbcan.2007.10.004. PMID: 18068131.
Article99. Carbonaro M, Escuin D, O’Brate A, Thadani-Mulero M, Giannakakou P. Microtubules regulate hypoxia-inducible factor-1α protein trafficking and activity: implications for taxane therapy. J Biol Chem. 2012; 287:11859–11869. DOI: 10.1074/jbc.M112.345587. PMID: 22367210. PMCID: 3320934.
Article100. Jiang X, Zhang D, Zhang H, Huang Y, Teng M. Role of Ran-regulated nuclear-cytoplasmic trafficking of pVHL in the regulation of microtubular stability-mediated HIF-1α in hypoxic cardiomyocytes. Sci Rep. 2015; 5:9193. DOI: 10.1038/srep09193. PMID: 25779090. PMCID: 4361876.
Article101. Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009; 10:854–865. DOI: 10.1038/nrm2804. PMID: 19935668. PMCID: 3394690.
Article102. Schroeder CM, Vale RD. Assembly and activation of dynein-dynactin by the cargo adaptor protein Hook3. J Cell Biol. 2016; 214:309–318. DOI: 10.1083/jcb.201604002. PMID: 27482052. PMCID: 4970328.
Article103. Olenick MA, Tokito M, Boczkowska M, Dominguez R, Holzbaur EL. Hook adaptors induce unidirectional processive motility by enhancing the dynein-dynactin interaction. J Biol Chem. 2016; 291:18239–18251. DOI: 10.1074/jbc.M116.738211. PMID: 27365401. PMCID: 5000072.
Article104. Splinter D, Razafsky DS, Schlager MA, Serra-Marques A, Grigoriev I, Demmers J, Keijzer N, Jiang K, Poser I, Hyman AA, Hoogenraad CC, King SJ, Akhmanova A. BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol Biol Cell. 2012; 23:4226–4241. DOI: 10.1091/mbc.e12-03-0210. PMID: 22956769. PMCID: 3484101.
Article105. Lee HJ, Jung YH, Oh JY, Choi GE, Chae CW, Kim JS, Lim JR, Kim SY, Lee SJ, Seong JK, Han HJ. BICD1 mediates HIF1α nuclear translocation in mesenchymal stem cells during hypoxia adaptation. Cell Death Differ. 2018; DOI: 10.1038/s41418-018-0241-1. PMID: 30464225. [Epub ahead of print].
Article106. Depping R, Steinhoff A, Schindler SG, Friedrich B, Fagerlund R, Metzen E, Hartmann E, Köhler M. Nuclear translocation of hypoxia-inducible factors (HIFs): involvement of the classical importin α/β pathway. Biochim Biophys Acta. 2008; 1783:394–404. DOI: 10.1016/j.bbamcr.2007.12.006. PMID: 18187047.
Article107. Miyamoto Y, Yamada K, Yoneda Y. Importin α: a key molecule in nuclear transport and non-transport functions. J Biochem. 2016; 160:69–75. DOI: 10.1093/jb/mvw036. PMID: 27289017.
Article108. Hutten S, Flotho A, Melchior F, Kehlenbach RH. The Nup358-RanGAP complex is required for efficient importin α/β-dependent nuclear import. Mol Biol Cell. 2008; 19:2300–2310. DOI: 10.1091/mbc.e07-12-1279. PMID: 18305100. PMCID: 2366868.
Article109. Hamada M, Haeger A, Jeganathan KB, van Ree JH, Malureanu L, Wälde S, Joseph J, Kehlenbach RH, van Deursen JM. Ran-dependent docking of importin-β to RanBP2/Nup358 filaments is essential for protein import and cell viability. J Cell Biol. 2011; 194:597–612. DOI: 10.1083/jcb.201102018. PMID: 21859863. PMCID: 3160583.
Article110. Wälde S, Thakar K, Hutten S, Spillner C, Nath A, Rothbauer U, Wiemann S, Kehlenbach RH. The nucleoporin Nup358/RanBP2 promotes nuclear import in a cargo-and transport receptor-specific manner. Traffic. 2012; 13:218–233. DOI: 10.1111/j.1600-0854.2011.01302.x. PMID: 21995724.
Article111. Chachami G, Paraskeva E, Mingot JM, Braliou GG, Görlich D, Simos G. Transport of hypoxia-inducible factor HIF-1α into the nucleus involves importins 4 and 7. Biochem Biophys Res Commun. 2009; 390:235–240. DOI: 10.1016/j.bbrc.2009.09.093. PMID: 19788888.
Article112. Tazat K, Schindler S, Depping R, Mabjeesh NJ. Septin 9 isoform 1 (SEPT9_i1) specifically interacts with importin-α 7 to drive hypoxia-inducible factor (HIF)-1α nuclear translocation. Cytoskeleton (Hoboken). 2018; DOI: 10.1002/cm.21450. PMID: 29742803. [Epub ahead of print].113. Takata M, Shimamoto S, Yamaguchi F, Tokuda M, Tokumitsu H, Kobayashi R. Regulation of nuclear localization signal-importin α interaction by Ca2+/S100A6. FEBS Lett. 2010; 584:4517–4523. DOI: 10.1016/j.febslet.2010.09.052. PMID: 20965181.
Article114. Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci U S A. 1996; 93:12969–12973. DOI: 10.1073/pnas.93.23.12969. PMID: 8917528. PMCID: 24030.115. Ebert BL, Bunn HF. Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein. Mol Cell Biol. 1998; 18:4089–4096. DOI: 10.1128/MCB.18.7.4089. PMID: 9632793. PMCID: 108993.
Article116. Ruas JL, Poellinger L, Pereira T. Functional analysis of hypoxia-inducible factor-1 α-mediated transactivation. Identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein. J Biol Chem. 2002; 277:38723–38730. DOI: 10.1074/jbc.M205051200. PMID: 12133832.
Article117. Ruas JL, Poellinger L, Pereira T. Role of CBP in regulating HIF-1-mediated activation of transcription. J Cell Sci. 2005; 118:301–311. DOI: 10.1242/jcs.01617. PMID: 15615775.
Article118. Kasper LH, Boussouar F, Boyd K, Xu W, Biesen M, Rehg J, Baudino TA, Cleveland JL, Brindle PK. Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. EMBO J. 2005; 24:3846–3858. DOI: 10.1038/sj.emboj.7600846. PMID: 16237459. PMCID: 1283945.
Article119. Perez-Perri JI, Dengler VL, Audetat KA, Pandey A, Bonner EA, Urh M, Mendez J, Daniels DL, Wappner P, Galbraith MD, Espinosa JM. The TIP60 complex is a conserved coactivator of HIF1A. Cell Rep. 2016; 16:37–47. DOI: 10.1016/j.celrep.2016.05.082. PMID: 27320910. PMCID: 4957981.
Article120. Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011; 145:732–744. DOI: 10.1016/j.cell.2011.03.054. PMID: 21620138. PMCID: 3130564.
Article121. Schoepflin ZR, Silagi ES, Shapiro IM, Risbud MV. PHD3 is a transcriptional coactivator of HIF-1α in nucleus pulposus cells independent of the PKM2-JMJD5 axis. FASEB J. 2017; 31:3831–3847. DOI: 10.1096/fj.201601291R. PMID: 28495754. PMCID: 5572688.
Article122. Gabriely G, Wheeler MA, Takenaka MC, Quintana FJ. Role of AHR and HIF-1α in glioblastoma metabolism. Trends Endocrinol Metab. 2017; 28:428–436. DOI: 10.1016/j.tem.2017.02.009. PMID: 28318896. PMCID: 5438779.
Article123. Lu H, Chen I, Shimoda LA, Park Y, Zhang C, Tran L, Zhang H, Semenza GL. Chemotherapy-induced Ca2+ release stimulates breast cancer stem cell enrichment. Cell Rep. 2017; 18:1946–1957. DOI: 10.1016/j.celrep.2017.02.001. PMID: 28228260.
Article124. Pawlus MR, Wang L, Hu CJ. STAT3 and HIF1α cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene. 2014; 33:1670–1679. DOI: 10.1038/onc.2013.115. PMID: 23604114. PMCID: 3868635.
Article125. Villa JC, Chiu D, Brandes AH, Escorcia FE, Villa CH, Maguire WF, Hu CJ, de Stanchina E, Simon MC, Sisodia SS, Scheinberg DA, Li YM. Nontranscriptional role of Hif-1α in activation of γ-secretase and notch signaling in breast cancer. Cell Rep. 2014; 8:1077–1092. DOI: 10.1016/j.celrep.2014.07.028. PMID: 25131208. PMCID: 4346175.
Article126. Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014; 14:801–814. DOI: 10.1038/nrc3846. PMID: 25568920. PMCID: 4401080.
Article127. Quintana FJ, Sherr DH. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol Rev. 2013; 65:1148–1161. DOI: 10.1124/pr.113.007823. PMID: 23908379. PMCID: 3799235.
Article128. Finlay DK, Rosenzweig E, Sinclair LV, Feijoo-Carnero C, Hukelmann JL, Rolf J, Panteleyev AA, Okkenhaug K, Cantrell DA. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012; 209:2441–2453. DOI: 10.1084/jem.20112607. PMID: 23183047. PMCID: 3526360.
Article129. Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, Pan F, Priel A, Clish CB, Robson SC, Quintana FJ. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015; 21:638–646. DOI: 10.1038/nm.3868. PMID: 26005855. PMCID: 4476246.
Article130. Blouin CC, Pagé EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1α. Blood. 2004; 103:1124–1130. DOI: 10.1182/blood-2003-07-2427. PMID: 14525767.
Article131. Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, Gonzalez FJ, Ikuta T, Kawajiri K, Fujii-Kuriyama Y. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol. 2009; 29:6391–6400. DOI: 10.1128/MCB.00337-09. PMID: 19822660. PMCID: 2786870.
Article132. Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011; 478:197–203. DOI: 10.1038/nature10491. PMID: 21976023.
Article133. Martinez VG, Ontoria-Oviedo I, Ricardo CP, Harding SE, Sacedon R, Varas A, Zapata A, Sepulveda P, Vicente A. Overexpression of hypoxia-inducible factor 1 α improves immunomodulation by dental mesenchymal stem cells. Stem Cell Res Ther. 2017; 8:208. DOI: 10.1186/s13287-017-0659-2. PMID: 28962641. PMCID: 5622468.